* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Does dispersal capacity matter for freshwater biodiversity under
Effects of global warming on human health wikipedia , lookup
Climate engineering wikipedia , lookup
Citizens' Climate Lobby wikipedia , lookup
Climate governance wikipedia , lookup
Attribution of recent climate change wikipedia , lookup
Economics of global warming wikipedia , lookup
Climate sensitivity wikipedia , lookup
Solar radiation management wikipedia , lookup
Climate change adaptation wikipedia , lookup
Climate change in Tuvalu wikipedia , lookup
Media coverage of global warming wikipedia , lookup
Scientific opinion on climate change wikipedia , lookup
Effects of global warming wikipedia , lookup
General circulation model wikipedia , lookup
Climate change and agriculture wikipedia , lookup
Public opinion on global warming wikipedia , lookup
Effects of global warming on humans wikipedia , lookup
Climate change and poverty wikipedia , lookup
Surveys of scientists' views on climate change wikipedia , lookup
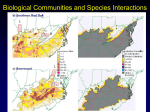
Freshwater Biology (2016) doi:10.1111/fwb.12874 Does dispersal capacity matter for freshwater biodiversity under climate change? ALEX BUSH*,† AND ANDREW J. HOSKINS‡ *Macquarie University, Sydney, NSW, Australia † Canadian Rivers Institute, University of New Brunswick, Fredericton, NB, Canada ‡ CSIRO Land and Water, Canberra, ACT, Australia SUMMARY 1. Freshwater ecosystems appear to be sensitive to even minor climatic shifts, and the dendritic nature of rivers as well as patchy distribution of habitats within the terrestrial landscape could limit the ability of species to track suitable climate conditions. Although the importance of dispersal is recognised in theory, there is great uncertainty when quantifying the capacity of species to shift their distributions in response to climate change. 2. The influence of dispersal capacity on species’ vulnerability to climate change was assessed, using the modelled projections of 527 freshwater species in New South Wales (NSW), Australia. Species’ future ranges were calculated by iteratively identifying colonisation of accessible habitats and loss of suitable habitats within network models. The accessibility of new habitats was based on a given dispersal mode (aquatic, semi-terrestrial and aerial). The relative impact of dispersal parameters on projected range were evaluated alongside other known sources of uncertainty (climate and emissions scenarios, modelling algorithm and biological group), analysed collectively in a generalised additive mixed-model, and spatially to locate regions of NSW where projections are associated with the most uncertainty. 3. Our simulations (1.4 million scenario combinations) suggest at least a third of species will lose more than half their range under climate change. Nevertheless, we emphasise the broad uncertainty that any average encapsulates. Dispersal capacity only had a minor impact on projected range shifts relative to other modelling assumptions but the network-pathways and maps of uncertainty have value for conservation planning at large scales. Projected range losses initially decreased rapidly as dispersal rates increased but the benefits are reduced above 2–3 km year 1. Taxa restricted to dispersal within the stream network (aquatic) were more vulnerable to climate change than taxa with semi-terrestrial or aerial dispersal and maps of variation due to dispersal mode and rate indicate where habitat connectivity would be most beneficial. 4. This study demonstrates the breadth of uncertainties that challenge plans for improving ecosystem adaptation under climate change and highlights where in the landscape those differences were consistent. We emphasise the need for freshwater conservation studies to be ecologically representative, to focus on broad-scale connectivity for taxa that can move between catchments, and an accessible network of refugia for taxa with more limited dispersal. Keywords: environmental niche model, generalised additive mixed-effects models, graph network, species distribution modelling, uncertainty Introduction There have been significant declines in global freshwater biodiversity as a result of the intensive and growing exploitation of water resources, flow modification, habitat destruction, pollution and introduced species (Allen et al., 1999; Dudgeon et al., 2006). Although it is very likely these stressors will continue to be the dominant threats to freshwater ecosystems (V€ or€ osmarty et al., 2010; Kuemmerlen et al., 2015b), climate change will Correspondence: Alex Bush, Canadian Rivers Institute, University of New Brunswick, Fredericton, E3B 5A3 NB, Canada. E-mail: alexalbush@ gmail.com © 2016 John Wiley & Sons Ltd 1 2 A. Bush & A. J. Hoskins have significant additional impacts across entire landscapes (Dudgeon, 2010; Kingsford, 2011). As a result, management agencies are increasingly aware that actions will be required to aid adaptation of biodiversity under climate change and avoid local extinctions (Lukasiewicz, Finlayson & Pittock, 2013, 2014). However, the merits of particular management actions are difficult to assess due to the uncertainty involved when assessing vulnerability to climate change (Buisson et al., 2010; Beale & Lennon, 2012). This study examines the factors influencing projected outcomes in New South Wales (NSW) Australia. Species distribution models (SDMs) are one of the most prevalent methods being used to assess the potential impacts of climate change on species and guide conservation planning (Wiens et al., 2009; Kujala et al., 2013; Maggini et al., 2013). However, many decisions in the modelling process can influence outcomes, including the choices of environmental variables used (Synes & Osborne, 2011), presence/absence threshold (Liu, White & Newell, 2013), modelling algorithm (Elith et al., 2006), model complexity (Warren et al., 2011), and for future climate data, the emission scenario and global climate model (GCM) used (Buisson & Grenouillet, 2010; James et al., 2013). In addition, an important limitation of most studies is that realistic rates and modes of dispersal are not accounted for (Saupe et al., 2012), and therefore projections indicate the overall shift in favourable climates, but not the extent to which these would be accessible (Reside, Vanderwal & Kutt, 2012). Model training is also heavily influenced by the geographic extent of the analysis which should reflect the region available to the species (Elith, Kearney & Phillips, 2010), and therefore relates to the assumptions behind species’ dispersal capacity (Barbet-Massin et al., 2012). As there is no universal agreement on an optimal modelling strategy, users are strongly advised to be mindful of model uncertainty (Wiens et al., 2009) but without being quantified, multiple sources of potential error can contribute to a sense of mounting uncertainty in all projections posing challenges for decision-making. Thus, it is important to understand which factors are most influential, so we can assess confidence in a particular model, and whether the spatial distribution of uncertainty could potentially alter preferred management actions (Kujala et al., 2013; Wright et al., 2015). As the availability of fine-resolution geographic data relevant to freshwater ecosystems has increased (e.g. hydrology or fine scale topography), the number of SDM applications to freshwater taxa has rapidly expanded (Elith et al., 2011; Kuemmerlen, Petzoldt & Domisch, 2015a). However, a number of challenges remain, such as correcting for patchy and biased species records within the nested spatial structure of river systems and combining information from multiple sources at different temporal and spatial scales (Domisch et al., 2015). Large-scale distribution patterns such as shifts in species ranges due to climate change can be approximated by broad-scale predictors (Comte et al., 2012; Domisch et al., 2012; Simaika et al., 2013), but species ranges can still be overestimated if modelling decisions and environmental variables are not appropriate to freshwater systems (Domisch et al., 2013). Dispersal is a particularly important process in freshwater systems due to their patchy or structured distribution (Heino, 2011; Gr€ onroos et al., 2013). Over shorter time-scales, dispersal capacity limits the successful recolonization of sites following restoration (Tonkin et al., 2014), and has clear implications for taxa expected to expand their ranges under climate change (Hein, € Ohlund & Englund, 2011; Comte et al., 2012). Unfortunately, quantitative estimates of dispersal capacity are lacking for most species meaning their ability to track shifting climates in the future remains highly uncertain (Driscoll et al., 2014). This study aims to establish whether dispersal capacity in freshwater taxa is likely to affect management planning decisions by identifying (i) what differences in dispersal capacity have the greatest impact on their vulnerability to climate change, (ii) how important dispersal capacity is likely to be versus other sources of uncertainty in model projections, and (iii) where different sources of uncertainties are concentrated? In Australia, significant temperature increases and reductions in rainfall (Hobday & Lough, 2011) are likely to alter habitat suitability for many species and a number of studies have applied SDMs to freshwater taxa, including fish (Bond et al., 2011), crayfish (James et al., 2013), and Odonata (Bush et al., 2014). The capacity for freshwater communities to naturally adapt to climate change is a particular concern in New South Wales because the coastal rivers flow eastwards and do not encompass a large latitudinal range that may restrict movement to track suitable climatic conditions (Turak et al., 2011; Bush et al., 2012). We employ a network-flow approach inspired by Williams et al. (2005) to track suitable dispersal pathways under changing environmental conditions. The process was repeated, using a variety of modelling assumptions (emissions scenarios, climate models, modelling algorithms, dispersal rates and dispersal mode) to examine the influence of each assumption on the extent of ‘accessible’ future habitat. Finally, © 2016 John Wiley & Sons Ltd, Freshwater Biology, doi: 10.1111/fwb.12874 Dispersal ability and projected impacts of climate change 3 to identify biodiversity patterns that are not biased spatially due to taxonomy, we applied the analysis to 527 species across a wide variety of ecological groups that also represent the breadth of dispersal modes present. Methods Species data The study used occurrence records collated from a wide range of sources, including state monitoring programs, museum collections, federal government survey records, local catchment authorities, scientific literature and private collectors (see Appendix S1). The final assessment included models of 109 species of Odonata, 62 species of Hemiptera, 92 frogs, 44 crayfish, 57 fish, 118 aquatic or semi-aquatic plants and 45 freshwater molluscs. Modelled species represented a high proportion of the group’s regional diversity (83–100%), although plants (c. 70%) and molluscs (c. 20%) were less complete. The primary reason for including many species was to account for differences in habitat requirements and range sizes, and to reduce the effect of taxonomic bias on the estimated spatial distribution of biodiversity. We would have liked to include a broader range of invertebrate taxa but major biases in their collection and recording meant they were unsuitable for spatial modelling. The study focused on species with at least one record within an NSW catchment, including those catchments in which the state has a management role (Snowy, Genoa, and Murray–Darling) (Fig. 1). Each species modelled also needed to have been recorded in 15 or more sub-catchments in Australia. Although the majority of records were collected within the last 20 years (95%), records as far back as 1960 were also used if native vegetation at those sites was still intact, and the species had not been recorded in more than 14 sub-catchments more recently. Environmental data Organising the modelling environment and predictor variables to reflect the structure of a freshwater system is important when predicting habitat suitability because it can influence the accuracy of freshwater SDMs without necessarily affecting performance metrics (Domisch et al., 2013). Therefore, rather than using gridded data, models were based on the stream network from the Australian Hydrological Geospatial Fabric (Geofabric, 2011) that included upstream and downstream connectivity, catchment topography and geological composition. Climatic and hydrological parameters (mean Fig. 1 Point density of occurrence data in Australia for freshwater species found in New South Wales, including the boundaries of the study catchments. annual flow, dry-season length) as well as forecast for each parameter under climate change were provided at the same scale by James et al. (2013). Finally, given bivalve larvae may be more dependent on the community of fish present than the environmental conditions themselves, we added a species-specific estimate of fish community dissimilarity (Lois et al., 2014). Using the projected habitat suitability of all modelled fish, the mean dissimilarity (Bray–Curtis Index) was calculated between each location and all recorded sites. The importance of fish community dissimilarity to a bivalve’s distribution thus depended on whether fish communities were similar among recorded sites, and whether they differed at the locations of pseudo-absences. To compare the variation among projections driven by choice of climate models, future projections included a wide range of plausible future scenarios. Selecting multiple GCMs can span the range of uncertainty in predicting future climates (Buisson et al., 2010), but some studies suggest climate ensembles can perform better simulating observed conditions (Fordham, Wigley & Brook, 2011; Fordham et al., 2012). Therefore, in addition to a seven-GCM mean ensemble that had performed well for the region (Fordham et al., 2011), four further GCMs were chosen based on their predictive performance in south-east Australia (Evans & Ji, 2012); MIROC 3.2 (med), MPI-ECHAM 5, CCCMA CGCM 3.1 and CSIRO MK3.0. The climate change projections were based on two representative concentration pathways (RCPs; Van Vuuren et al., 2011) that describe a medium © 2016 John Wiley & Sons Ltd, Freshwater Biology, doi: 10.1111/fwb.12874 4 A. Bush & A. J. Hoskins and a high emissions scenario that result in radiative forcing reaching 6 and 8.5 W m 2, respectively, by 2100, equivalent to global average temperatures increasing 3.0 and 4.9 °C, respectively (Rogelj, Meinshausen & Knutti, 2012). Lower emissions scenarios were considered unlikely (Peters et al., 2013). Species distribution models Species distributions were modelled, using five common algorithms: generalised linear models (GLM), generalised boosted models (GBM), generalised additive models (GAM), multivariate adaptive regression splines (MARS) and Maxent (Elith et al., 2006). Algorithms were fitted, using 10 replicates for cross-validation using a standard 70:30 split, weighting pseudo-absences and presences equally, and using the default settings in the R packages dismo (Hijmans et al., 2013) and biomod2 (Thuiller et al., 2009). The true skills statistic was used for model evaluation (Allouche, Tsoar & Kadmon, 2006) and to weight ensemble projections. To avoid overfitting, the location of up to 10 000 pseudo-absences were chosen from the range of environments historically accessible to that species (Barbet-Massin et al., 2012) based on their dispersal mode. Pseudo-absence were selected at random, but weighted by density of other species records in the same group to account for sampling bias. For the purposes of model fitting, species were split into three broad modes: ‘aquatic’ taxa dependent on standing water (fish and molluscs), ‘semi-terrestrial’ taxa with the some capacity to disperse overland (crayfish, frogs), and ‘aerial’ taxa with either passive or active aerial dispersal (plants and insects). For ‘aerial’ groups, we used the results of a previous study, that modelled odonates, which suggested pseudo-absences be excluded from sub-catchments more than 300 km from occurrence records (Bush et al., 2014). Pseudo-absences for ‘semiterrestrial’ species were also selected within 300 km of occurrence records, and from within the same catchments as occurrence records for ‘aquatic’ species (see description of connecting pathways below). Although restricting the extent of pseudo-absences improves model sensitivity within a species range, it also increases the likelihood models can extrapolate beyond species– environment relationships (Vanderwal et al., 2009). To limit extrapolation, projected suitability was reduced if conditions occurred beyond the environmental limits of the training data, either by 50% if extrapolation was necessary for only one parameter, or reduced to zero if conditions of multiple parameters extended beyond the ranges of the training data (Elith et al., 2011). Predictor variables were selected using the Akaike Information Criterion (AIC) (Warren & Seifert, 2011) to complement a set of three parameters considered central to environmental suitability; one thermal (typically maximum or minimum temperature), one rainfall (typically precipitation seasonality) and one hydrological (mean annual flow). After removing strongly correlated factors (>0.7), additional parameters were added through forward selection. Selected variables and model statistics are provided for all species in Appendices S2 and S3. Dispersal rate, mode and connectivity When assessing the vulnerability of freshwater taxa to climate change, there is a clear need to distinguish between species with differences in dispersal ecology as this is expected to affect both model fitting, and capacity to track climatic shifts. Straight-line measurements of the distances separating sites (i.e. Euclidean) are often used for convenience but do not reflect species’ habitat requirements or dispersal pathways (Grant et al., 2010), a feature especially true of structured landscapes such as river networks (Sutherland, Fuller & Royle, 2014). To manage this issue, the distances of each sub-catchment to all others within a 75 km radius were described based on three dispersal modes: ‘aquatic’, ‘semi-terrestrial’ and ‘aerial’. Naturally the processes driving passive and active dispersal are likely to differ, and the movement of some aquatic insects and plants for instance may be better reflected by stream-distances. Whilst in some cases, it may be possible to use more specific dispersal traits (Radinger & Wolter, 2014), this is not true of all biological groups (Angert et al., 2011) and therefore dispersal modes had to be generalised for each biological group to define physical limits to the training environment (‘background-mode’). The ‘aquatic’ dispersal mode measured distances between nodes of the stream network, calculated in R using the package igraph (Csardi & Nepusz, 2006). Stream network distances maximised the distances between headwaters within a catchment, and disconnect sites from sub-catchments in neighbouring catchments. However, diadromous fish were allowed to move to new river catchments whose outflow was within 200 km of an occupied catchment’s river mouth; given this was the minimum distance populations of some species must have dispersed to colonise Tasmania (Hammer et al., 2014). Upstream movements were considered as likely as downstream but prevented where the location of natural barriers such as waterfalls was known (1312 listed in eastern Australia; Geofabric, 2011). The exceptions © 2016 John Wiley & Sons Ltd, Freshwater Biology, doi: 10.1111/fwb.12874 Dispersal ability and projected impacts of climate change were five species of fish (Anguilla australis and A. reinhardtii, Galaxias brevipinnis, Gobiomorphus coxii and Mordacia mordax) that are able to navigate around or over river barriers (Raadik, 2013). For the purposes of this study, artificial barriers were not included because the data available did not cover the majority of eastern Australia. Distances moved in the ‘semi-terrestrial’ dispersal mode also followed the stream network, but further connections were added to catchment headwaters. Species could therefore move between adjacent catchments, as well as between headwaters within catchments separated by large distances along the stream network. Multiple headwater crossings were possible but, to emphasise their lower likelihood and moderate the training extent of some species, the real distance of these connections was increased by 25 times. Lastly, the connectivity among sub-catchments for ‘aerial’ species simply used the straight-line distance between sub-catchments. Modelling dispersal pathways in future time intervals As originally demonstrated by Williams et al. (2005), dispersal chains were calculated for each species based on connections between occupied sub-catchments at successive future time-slices (each decade from 2025 to 2085). To begin with, the projected habitat suitability of current and future conditions for a species, using a given SDM algorithm, GCM and RCP were transformed to presence/absence maps of a species’ potential range. Thresholds were selected to optimise the true skill statistic based on the review by Liu et al. (2013). Starting from the occurrence records used to fit the SDM, other suitable sub-catchments were added to the species’ range if they were accessible from those locations given the designated mode and rate of dispersal (after examining the range spread, annual rates were aggregated to a 10-year period to reduce computation time). This process was repeated 10 times to establish a historic current range equivalent to 100 years, before introducing climate change projections. Thereafter, the distribution of suitable habitat was replaced by the projection for 2025, and after allowing new sub-catchments to be colonised, occupied cells that had become environmentally unsuitable were subtracted from the species’ range. This process was repeated for each decade from 2025 to 2085 before recording the final range. Given the uncertainty regarding species dispersal capacity, we simulated range shifts based on 45 different dispersal scenarios (three dispersal modes and 15 different rates: 0.5–7.5 km year 1 in 0.5 km increments). To 5 account for other sources of uncertainty, all dispersal scenarios were repeated for each combination of modelling algorithm (n = 6), emissions scenario (n = 2) and climate model (n = 5); a total of 2700 runs per species, more than 1.4 million in total. The code used to run this analysis in R is provided in Appendix S4. The variation in species’ final occurrence was calculated as the average difference in the occupancy of each treatment, accounting for differences in the number of species modelled when comparing biological groups. Data analyses Given the nested nature of the data and the nonlinear impact of dispersal rate, generalised additive mixedeffects models (GAMM; via the R package mgcv; Wood, 2004), using a Gaussian error distribution and identity link, were used to test how much the rate of dispersal affected species’ projected vulnerability (percentage change in extent), and the potential for this effect to differ among dispersal modes (Wood & Scheipl, 2014). Variation in projected range due to dispersal was placed in the context of alternative sources of uncertainty by including the GCM and SDM algorithm as random effects, and RCP. Individual smooth terms were fitted to dispersal rate grouped by dispersal mode, giving a unique smooth function for each of the three dispersal modes being assessed. An additional factor ‘background-mode’ was used to specify the assumed mode of dispersal that controlled selection of pseudo-absences. We also added current range size and altitude as fixed effects given their known influence on species’ vulnerability to climate change and to account for inter-specific variation. Unfortunately, with so many factors and dispersal combinations, computational limitations meant GAMMs could not be fitted for the whole dataset. Testing with subsets did not show a significant additional effect of ‘background-mode’ and the effect of biological group was extremely weak so models were fitted to each biological group separately (see Appendix S5). Model selection was based on the AIC and relative variable importance was estimated from marginal and conditional R2 (Nakagawa & Schielzeth, 2013). Results Climate change is likely to become a major threat to freshwater biodiversity in NSW, although we emphasise the precise degree of threat projected depends on numerous factors. For example, averaged across all © 2016 John Wiley & Sons Ltd, Freshwater Biology, doi: 10.1111/fwb.12874 6 A. Bush & A. J. Hoskins combinations, projected range losses were >50% used MPI-ECHAM 5, but only 7% using MIROC 3.2 (med). Changes in range size also tended to be greatest in species with smaller current ranges, which were more common among the crayfish and frogs, and to a lesser extent Odonata (Fig. 2). Dispersal mode and rate also had a significant impact on range losses. Averaged across climate scenarios and SDM algorithms, the ranges of 231 species (44%) were projected to decline by over 50% by 2085 (RCP 8.5) based on dispersal rates of just 0.5 km year 1, but was reduced to 39% at 2 km year 1, and 35% at 7.5 km year 1. More than 40% of species lost all suitable habitat in at least one projected scenario. Given the breadth of climate scenarios, and potential limiting dispersal constraints, few species could be expected to consistently expand their ranges under climate change, but if each scenario was considering equally likely then at least 26 species were more likely to expand their range rather than decline. Inevitably the GAMM analysis confirmed all the factors being varied in each projection had a significant effect on the projected future change in a species range. Naturally higher rates of dispersal enabled range declines to be offset in more cases, but those benefits diminished rapidly and dispersal rate had little further effect above 2–3 km year 1. A similar relationship was Fig. 2 Summary of the projected change to the range of all 527 species modelled in this study at three rates of dispersal 0.5, 2 and 7.5 km year 1. Colours indicate the number of projections, out of 180 for each species/dispersal rate combination, that predicted a particular range shift. © 2016 John Wiley & Sons Ltd, Freshwater Biology, doi: 10.1111/fwb.12874 Dispersal ability and projected impacts of climate change common to each dispersal mode (Fig. 3 and Appendix S5), but most prominent if taxa had ‘aerial’ dispersal. Range declines were not offset to the same extent when taxa were confined to ‘aquatic’ or even ‘semi-terrestrial’ dispersal pathways, although in the latter case higher dispersal rates continued to reduce range losses making the trend more linear. Although in some circumstances dispersal mode and rate could have a significant effect on the projected range of a species under climate change, their relative importance was relatively low (5 and 1.5%, respectively). By comparison inter-specific differences in current range were responsible for 32.5% of the variation, GCMs for 29%, current mean altitude 16%, modelling algorithm (a) (b) (c) Fig. 3 Predictions of the percentage change in the range of Odonata (green), crayfish (purple) and frogs (red) due to climate change by 2085 (RCP 8.5), in response to increasing rates of dispersal. Predictions are split between the three dispersal modes (a) aquatic, (b) semi-terrestrial and (c) aerial, and assume the same mean current range size and altitude. Colour intensity is weighted by the standard error of the generalised additive mixed-effects model predictions. Other biological groups were not plotted due to overlaps, but are available in Appendix S5. 7 9% and RCP 6.5%. The relative importance of modelling factors was consistent among biological groups, but varied most (6%) for current range size. Although many species could shift their distribution to higher elevations with just low rates of dispersal, species currently at higher altitudes were more vulnerable under climate change because the area of remaining suitable habitat declined rapidly. The GAMMs for crayfish and frogs suggest they are particularly vulnerable to climate change, which is likely to be because they tend to have smaller current ranges at higher elevations, and hence narrower environmental tolerances. The influence of modelling assumptions also showed different spatial patterns. For example, the projected range shifts of Euastacus spinifer (a parastacid spiny crayfish endemic to NSW) could vary greatly with the choice of GCM and SDM algorithm but there can also be very clear constraints placed by dispersal pathways, and at a finer scale still, by dispersal rates (Fig. 4). Whether catchment headwaters at higher elevations would remain suitable for E. spinifer was highly dependent on the projection GCM, whereas the suitability of coastal lowlands for this species varied depending on the modelling algorithm. Southern, and to some extent northern, range expansion was dependent on the dispersal mode and the ability to travel large distances across catchment boundaries. Finally, the greatest differences in occupancy due to the dispersal rate were not at expanding range margins, but around the perimeter of the current range. Though high rates of dispersal may be needed to access distant sites, this pattern was common because the dispersal rate was immaterial when on average those sub-catchments were not projected to be environmentally suitable. For NSW as a whole, climate change was projected to shift freshwater diversity east towards the more mesic coastal catchments, although gains were still possible at a number of locations in the south of the Murray– Darling (Fig. 5). The number of species that could persist at high elevations was heavily dependent on the GCM used, whereas the differences due to RCP and the modelling algorithm were more evenly distributed, and still reflecting the overall bias in richness regionally. However, the greatest differences in the richness of a particular area were contingent on which ecological group was used, especially along the coastal fringe. Given all these differences, those due to dispersal are interesting as they do not follow trends in richness. As with the maps of E. spinifer, the differences due to the dispersal mode indicate where taxa with dispersal limitations are most likely to be excluded from, and therefore this is most common © 2016 John Wiley & Sons Ltd, Freshwater Biology, doi: 10.1111/fwb.12874 8 A. Bush & A. J. Hoskins (a) (b) 1.0 1.0 0.8 0.8 0.6 0.6 0.4 0.4 0.2 0.2 0.0 0.0 (c) (d) 0.6 0.6 0.5 0.5 0.4 0.4 0.3 0.3 0.2 0.2 0.1 0.1 0.0 0.0 (e) (f) 0.6 0.6 0.5 0.5 0.4 0.4 0.3 0.3 0.2 0.2 0.1 0.1 0.0 0.0 Fig. 4 Mean occupancy of 1350 projections of Euastacus spinifer in sub-catchments of coastal New South Wales under a) current, and b) future climate conditions (2085 RCP 8.5). Catchment boundaries are marked in black. The following maps show the mean difference in projected future occupancy of E.spinifer due to choices of c) GCM (n=5), d) modelling algorithm (n=6), e) dispersal mode (n=3) and f) dispersal rate (n=15). © 2016 John Wiley & Sons Ltd, Freshwater Biology, doi: 10.1111/fwb.12874 Dispersal ability and projected impacts of climate change (a) (b) 9 (c) 120 120 100 100 80 80 60 60 40 40 20 20 0 0 40 20 0 −20 (d) (e) −40 (f) 0.15 0.15 0.15 0.10 0.10 0.10 0.05 0.05 0.05 0.00 0.00 0.00 (g) (h) (i) 0.15 0.15 0.15 0.10 0.10 0.10 0.05 0.05 0.05 0.00 0.00 0.00 Fig. 5 Mean occupancy of 2700 projections for each of 527 freshwater species modelled in catchments of New South Wales (outlined in black); under (a) current and (b) future climate conditions (2085), and (c) the difference between the (a) and (b). Maps (d–i) then show the mean difference in occupancy of each sub-catchment due to choices of (d) global climate model (n = 5), (e) representative concentration pathway (n = 2), (f) modelling algorithm (n = 6), (g) biological group (n = 7), (h) dispersal mode (n = 3) and (i) dispersal rate (n = 15). along the parts of the coast with multiple small separated catchments. Low mean differences in the projected ranges of taxa due to limitations of dispersal rate were present across the entire landscape, but do suggest that high rates of dispersal would be necessary to access the remaining suitable habitat across the different divisions of the Murray–Darling basin. Discussion This study showed that the dispersal capacity, in particular the dispersal mode, influences the outcome of species’ vulnerability assessments against a backdrop of many known uncertainties with modelling methods and climate projections (Buisson et al., 2010; Porfirio et al., © 2016 John Wiley & Sons Ltd, Freshwater Biology, doi: 10.1111/fwb.12874 10 A. Bush & A. J. Hoskins 2014; Wright et al., 2015). Naturally higher rates of dispersal could reduce the vulnerability of some taxa, but on average it only helped offset losses up to a rate of approximately 2–3 km year 1; with some further expansion possible in ‘semi-terrestrial’ dispersal scenarios. The inclusion of altitude in the GAMM, and moderate to severe projected range losses of many upland taxa, including crayfish (Mccormack, 2012), galaxiid fishes (Raadik, 2013) and Odonata (Bush et al., 2014), suggest that following suitable environmental conditions up altitudinal gradients cannot compensate for widespread declines. Likewise, higher dispersal capacity allowed taxa dispersing via ‘aquatic’ pathways to access more distant suitable habitats along stream networks, but the opportunity to offset losses elsewhere were limited by catchment boundaries (Morrongiello et al., 2011). Dispersal rate had a relatively minor influence on climate change projections because it moderated the area of suitable sub-catchments likely to be accessible regionally, an issue that is only important if suitable habitats are present in the first place and inherently dependent on other factors that affect the model globally like GCM and algorithm (Buisson et al., 2010; Wright et al., 2015). The GAMMs suggest range losses could be significantly reduced by advancing range shifts around 2–3 km year 1, well within the range already observed for some freshwater taxa in response to climate change (Hickling et al., 2006; Hassall, 2015). However, to be sustained for 70 years (2015–2085) would still require the taxa to shift the leading edge of their distribution between 140 and 210 km. This rate of dispersal also assumes shifts are maintained despite differences in landscape permeability and artificial barriers (Watling & Braga, 2015), information that could be easily incorporated into the dispersal-network model, but represented further assumptions beyond the scope of this study. As a result, there are potentially many taxa with low mobility that are likely to face severe declines due to climate change. For example, in a rare case where dispersal was directly recorded, using a chemical isotope on a stonefly in the USA for 2 years, no individuals were recorded more than 1 km from the stream channel (Macneale, Peckarsky & Likens, 2005). Unfortunately, this remains an isolated study and even generalising dispersal-related traits for well-known groups would be difficult (Angert et al., 2011). When considering future research, it is this uncertainty at the slow end of the dispersal spectrum that should be of greatest concern (Driscoll et al., 2014). Dispersal mode had a greater impact than dispersal rate on future projections because it both increased the area of accessible habitats and affected the distance a species had to travel to reach the same location. Selection of background points is important to the performance of SDMs under current conditions (Elith & Graham, 2009; Barbet-Massin et al., 2012), but ‘background-mode’ did not have a significant effect on projected range shifts relative to other major sources of uncertainty. Nonetheless, species’ occurrences should only be fitted against the range of environmental conditions accessible to them, which implies an assumption of dispersal pathways (Vanderwal et al., 2009; Domisch et al., 2013). However, in many cases, even the dispersal mode can be difficult to assign with confidence as the opportunities for movement may even vary between the different life-stages of a species (Driscoll et al., 2014). For example, less-mobile taxa like insects that lack aerial adults, molluscs, crayfish and hydrochorous plants could be transported long distances by waterbirds (Green, Figuerola & S anchez, 2002; Brochet et al., 2009; Van Leeuwen et al., 2012), and potentially track suitable climatic conditions through a series of long-distance jumps (Kelly et al., 2014). However, the prevalence of such events is unknown (Horv ath, Vad & Ptacnik, 2015), and most evidence in NSW suggests freshwater communities are spatially structured to some degree, particularly by catchment boundaries (Bush et al., 2012). Given the rapid rate of ongoing climate change, we must conservatively assume adaptive range shifts to offset range losses will be limited in freshwater taxa. Range declines are therefore inevitable, but early action to support the natural adaptation of biodiversity to changing conditions could improve the persistence of many species in the future. This study supports the need for assessments that plan conservation actions to be more representative and not depend on single groups or surrogates (Darwall et al., 2011). Furthermore, it is important more studies report the uncertainty surrounding climate change projections as the use of only select scenarios can be misleading (Beaumont, Hughes & Pitman, 2008). Furthermore, other aspects of SDM fitting such as the degree to which projections are allowed to extrapolate beyond their training environment (Elith et al., 2011), or the method for thresholding projected habitat suitability (Liu et al., 2013) could also be a significant source of uncertainty. Consensus on the most appropriate GCMs, modelling algorithm to use, or thresholding mechanism to apply to projected climate change impacts is unlikely in the near future, and therefore studies in other landscapes should also consider similar methods to partition sources of uncertainty and communicate their distribution. © 2016 John Wiley & Sons Ltd, Freshwater Biology, doi: 10.1111/fwb.12874 Dispersal ability and projected impacts of climate change The differences between projections due to uncertainty in the dispersal mode can help identify divisions between similar environments that may not pose a barrier to strong aerial taxa, but could be restored to improve connectivity for taxa with weaker flight or the capacity for semi-terrestrial movement (Alexander et al., 2011). One possible opportunity in NSW could be to add connectivity of freshwater communities to the objectives of the Great Eastern Ranges Initiative which has a predominantly terrestrial focus (Mackey, Watson & Worboys, 2010). Sub-catchments would not necessarily require formal protection, but could aid connectivity if by restoring riparian vegetation which also improves the quality of in-stream habitats, provides shade and buffers streams from adjacent land use (Capon et al., 2013). Based on the network of dispersal pathways, it is possible to identify the most efficient reserve system that protects species at each time interval (Phillips et al., 2008). However, as extensive as the reserve system in NSW is (Turak et al., 2011), the protection they offer freshwater species is questionable because reserve status does not address threats such as upstream water quality, modified flow regimes and invasive species (Stein & Nevill, 2011; Turak et al., 2011). To account for further assumptions in reserve design such as cost, as well as the value of possible alternative management actions was beyond the remit of this study. However, a strategic approach based on the dispersal network could be used in the future at smaller scales to identify preferred conservation corridors and complement policies for human climate adaptation (Lukasiewicz et al., 2013). Thirdly, if range shifts are likely to be limited by aquatic dispersal, or simply low movement rates, more information will be needed to identify the risks of persistence in situ under increasing climatic extremes (Leigh et al., 2014). Many fish for instance in NSW have wide environmental tolerances (Sternberg & Kennard, 2013) and only require standing water to be accessible in the landscape when conditions deteriorate (Chessman, 2013). Conversely many crayfish are restricted to high altitude refugia, isolated by unfavourable conditions at lower elevations (i.e. the ‘death valley’ scenario; Hughes, 2007; Hughes, Schmidt & Finn, 2009), and projected to lose all suitable habitat within their current range. Furthermore, regional range losses of more widespread species may not be trivial if they disguise the loss of genetically distinct populations and cryptic species in Australia (Balint et al., 2011; Faulks, Gilligan & Beheregaray, 2011; Hammer et al., 2014). Managers will need to consider whether 11 translocation is an option, or if climatic changes could be mitigated in natural and artificial refugia (Robson et al., 2013). Globally, freshwater ecosystems are suffering from multiple stressors (Dudgeon et al., 2006), and whilst short-term conservation management remains essential to slowing the pace of biodiversity loss, preparation for long-term sustainability under future conditions is becoming an increasing priority (Ormerod, 2009). The high spatial and temporal variability of freshwater systems in New South Wales presents a particularly challenging environment for biodiversity management, and the uncertainties become magnified when considering how ecosystems may respond under climate change (Ormerod, 2009). This study may appear to emphasise the uncertainties, but it is important to clearly recognise the assumptions that underpin projections and look for management options that are robust to uncertainty (Game et al., 2011). It is often easier to protect rather than restore important habitats and this should be considered as early as possible to aid the long-term adaptation of ecosystems. Acknowledgments This work was funded as part of the New South Wales Adaptation Research Hub. We are very grateful to all the institutions that provided records or environmental data as well as Renee Catullo, Dean Gilligan, Matthew Gordos, Hugh Jones, Rob McCormack, Dan Rosauer, Jeremy Vanderwal and Tom Weir. References Alexander L.C., Hawthorne D.J., Palmer M.A. & Lamp W.O. (2011) Loss of genetic diversity in the North American mayfly Ephemerella invaria associated with deforestation of headwater streams. Freshwater Biology, 56, 1456–1467. Allen A.P., Whittier T.R., Kaufmann P.R., Larsen D.P., O’connor R.J., Hughes R.M. et al. (1999) Concordance of taxonomic richness patterns across multiple assemblages in lakes of the northeastern United States. Canadian Journal of Fisheries and Aquatic Sciences, 56, 739–747. Allouche O., Tsoar A. & Kadmon R. (2006) Assessing the accuracy of species distribution models: prevalence, kappa and the true skill statistic (TSS). Journal of Applied Ecology, 43, 1223–1232. Angert A.L., Crozier L.G., Rissler L.J., Gilman S.E., Tewksbury J.J. & Chunco A.J. (2011) Do species’ traits predict recent shifts at expanding range edges? Ecology Letters, 14, 677–689. © 2016 John Wiley & Sons Ltd, Freshwater Biology, doi: 10.1111/fwb.12874 12 A. Bush & A. J. Hoskins Balint M., Domisch S., Engelhardt C.H.M., Haase P., Lehrian S., Sauer J. et al. (2011) Cryptic biodiversity loss linked to global climate change. Nature Climate Change, 1, 313–318. Barbet-Massin M., Jiguet F., Albert C.H. & Thuiller W. (2012) Selecting pseudo-absences for species distribution models: how, where and how many? Methods in Ecology and Evolution, 3, 327–338. Beale C.M. & Lennon J.J. (2012) Incorporating uncertainty in predictive species distribution modelling. Philosophical Transactions of the Royal Society B: Biological Sciences, 367, 247–258. Beaumont L.J., Hughes L. & Pitman A.J. (2008) Why is the choice of future climate scenarios for species distribution modelling important? Ecology Letters, 11, 1135–1146. Bond N., Thomson J., Reich P. & Stein J. (2011) Using species distribution models to infer potential climate changeinduced range shifts of freshwater fish in south-eastern Australia. Marine and Freshwater Research, 62, 1043–1061. Brochet A.L., Guillemain M., Fritz H., Gauthier-Clerc M. & Green A.J. (2009) The role of migratory ducks in the long-distance dispersal of native plants and the spread of exotic plants in Europe. Ecography, 32, 919–928. Buisson L. & Grenouillet G. (2010) Predicting the potential impacts of climate change on stream fish assemblages. American Fisheries Society Symposium, 73, 327–346. Buisson L., Thuiller W., Casajus N., Lek S. & Grenouillet G. (2010) Uncertainty in ensemble forecasting of species distribution. Global Change Biology, 16, 1145–1157. Bush A., Nipperess D., Turak E. & Hughes L. (2012) Determining vulnerability of stream communities to climate change at the landscape scale. Freshwater Biology, 57, 1689–1701. Bush A., Nipperess D.A., Theischinger G., Duursma D., Turak E. & Hughes L. (2014) Continental-scale assessment of risk to the Australian odonata from climate change. PLoS One, 9, e88958. Capon S., Chambers L., Mac Nally R., Naiman R., Davies P., Marshall N. et al. (2013) Riparian ecosystems in the 21st century: hotspots for climate change adaptation? Ecosystems, 16, 359–381. Chessman B.C. (2013) Identifying species at risk from climate change: traits predict the drought vulnerability of freshwater fishes. Biological Conservation, 160, 40–49. Comte L., Buisson L., Daufresne M. & Grenouillet G. (2012) Climate-induced changes in the distribution of freshwater fish: observed and predicted trends. Freshwater Biology, 58, 625–639. Csardi G. & Nepusz T. (2006) The igraph software package for complex network research. In: InterJournal, Complex Systems 1695. http://igraph.sf.net. Darwall W.R.T., Holland R.A., Smith K.G., Allen D., Brooks E.G.E., Katarya V. et al. (2011) Implications of bias in conservation research and investment for freshwater species. Conservation Letters, 4, 474–482. Domisch S., Ara ujo M.B., Bonada N., Pauls S.U., J€ahnig S.C. & Haase P. (2012) Modelling distribution in European stream macroinvertebrates under future climates. Global Change Biology, 19, 752–762. Domisch S., J€ahnig S., Simaika J.P., Kuemmerlen M. & Stoll S. (2015) Application of species distribution models in stream ecosystems: the challenges of spatial and temporal scale, environmental predictors and species occurrence data. Fundamental and Applied Limnology, 186, 45–61. Domisch S., Kuemmerlen M., J€ahnig S.C. & Haase P. (2013) Choice of study area and predictors affect habitat suitability projections, but not the performance of species distribution models of stream biota. Ecological Modelling, 257, 1–10. Driscoll D.A., Banks S.C., Barton P.S., Ikin K., Lentini P., Lindenmayer D.B. et al. (2014) The trajectory of dispersal research in conservation biology: systematic review. PLoS One, 9, e95053. Dudgeon D. (2010) Prospects for sustaining freshwater biodiversity in the 21st century: linking ecosystem structure and function. Current Opinion in Environmental Sustainability, 2, 422–430. Dudgeon D., Arthington A.H., Gessner M.O., Kawabata Z.I., Knowler D.J., Lev^eque C. et al. (2006) Freshwater biodiversity: importance, threats, status and conservation challenges. Biological Reviews, 81, 163–182. Elith J. & Graham C.H. (2009) Do they? How do they? WHY do they differ? on finding reasons for differing performances of species distribution models. Ecography, 32, 66–77. Elith J., Graham C.H., Anderson R.P., Dud~ak M., Ferrier S., Guisan A. et al. (2006) Novel methods improve prediction of species’ distributions from occurrence data. Ecography, 29, 129–151. Elith J., Kearney M. & Phillips S. (2010) The art of modelling range-shifting species. Methods in Ecology and Evolution, 1, 330–342. Elith J., Phillips S.J., Hastie T., Dud~ak M., Chee Y.E. & Yates C.J. (2011) A statistical explanation of MaxEnt for ecologists. Diversity and Distributions, 17, 43–57. Evans J.P. & Ji F. (2012) Choosing GCMs. NARCliM Technical Note 1. NARCliM Consortium, Sydney. Faulks L.K., Gilligan D.M. & Beheregaray L.B. (2011) The role of anthropogenic vs. natural in-stream structures in determining connectivity and genetic diversity in an endangered freshwater fish, Macquarie perch (Macquaria australasica). Evolutionary Applications, 4, 589–601. Fordham D.A., Wigley T.M.L. & Brook B.W. (2011) Multimodel climate projections for biodiversity risk assessments. Ecological Applications, 21, 3317–3331. Fordham D.A., Wigley T.M.L., Watts M.J. & Brook B.W. (2012) Strengthening forecasts of climate change impacts with multi-model ensemble averaged projections using MAGICC/SCENGEN 5.3. Ecography, 35, 4–8. © 2016 John Wiley & Sons Ltd, Freshwater Biology, doi: 10.1111/fwb.12874 Dispersal ability and projected impacts of climate change Game E.T., Lipsett-Moore G., Saxon E., Peterson N. & Sheppard S. (2011) Incorporating climate change adaptation into national conservation assessments. Global Change Biology, 17, 3150–3160. Geofabric (2011) Australian Hydrological Geospatial Fabric Data Product Specification – Surface Network Version 1.1.3. B.O. Meteorology, Australian Bureau of Meteorology. Grant E.H.C., Nichols J.D., Lowe W.H. & Fagan W.F. (2010) Use of multiple dispersal pathways facilitates amphibian persistence in stream networks. Proceedings of the National Academy of Sciences of the United States of America, 107, 6936–6940. Green A.J., Figuerola J. & Sanchez M.I. (2002) Implications of waterbird ecology for the dispersal of aquatic organisms. Acta Oecologica-International Journal of Ecology, 23, 177–189. Gr€ onroos M., Heino J., Siqueira T., Landeiro V.L., Kotanen J. & Bini L.M. (2013) Metacommunity structuring in stream networks: roles of dispersal mode, distance type, and regional environmental context. Ecology and Evolution, 3, 4473–4487. Hammer M.P., Unmack P.J., Adams M., Raadik T.A. & Johnson J.B. (2014) A multigene molecular assessment of cryptic biodiversity in the iconic freshwater blackfishes (Teleostei: Percichthyidae: Gadopsis) of south-eastern Australia. Biological Journal of the Linnean Society, 111, 521–540. Hassall C. (2015) Odonata as candidate macroecological barometers for global climate change. Freshwater Science, 34, 1040–1049. € Hein C.L., Ohlund G. & Englund G. (2011) Dispersal through stream networks: modelling climate-driven range expansions of fishes. Diversity and Distributions, 17, 641– 651. Heino J. (2011) A macroecological perspective of diversity patterns in the freshwater realm. Freshwater Biology, 56, 1703–1722. Hickling R., Roy D.B., Hill J.K., Fox R. & Thomas C.D. (2006) The distributions of a wide range of taxonomic groups are expanding polewards. Global Change Biology, 12, 450–455. Hijmans R.J., Phillips S., Leathwick J. & Elith J. (2013) dismo: Species Distribution Modeling. R package version 0.9-3. http://CRAN.R-project.org/package=dismo. Hobday A.J. & Lough J.M. (2011) Projected climate change in Australian marine and freshwater environments. Marine and Freshwater Research, 62, 1000–1014. Horv ath Z., Vad C.F. & Ptacnik R. (2015) Wind dispersal results in a gradient of dispersal limitation and environmental match among discrete aquatic habitats. Ecography, 39, 726–732. Hughes J.M. (2007) Constraints on recovery: using molecular methods to study connectivity of aquatic biota in rivers and streams. Freshwater Biology, 52, 616–631. 13 Hughes J.M., Schmidt D.J. & Finn D.S. (2009) Genes in streams: using DNA to understand the movement of freshwater fauna and their riverine habitat. BioScience, 59, 573–583. James C., Vanderwal J., Capon S., Hodgson L., Waltham N., Ward D. et al. (2013) Identifying Climate Refuges for Freshwater Biodiversity Across Australia. National Climate Change Adaptation Research Facility, Gold Coast. Kelly R., Lundy M.G., Mineur F., Harrod C., Maggs C.A., Humphries N.E. et al. (2014) Historical data reveal power-law dispersal patterns of invasive aquatic species. Ecography, 37, 581–590. Kingsford R.T. (2011) Conservation management of rivers and wetlands under climate change – a synthesis. Marine and Freshwater Research, 62, 217–222. Kuemmerlen M., Petzoldt T. & Domisch S. (2015a) Ecological models in freshwater ecosystems. Fundamental and Applied Limnology, 186, 1–3. Kuemmerlen M., Schmalz B., Cai Q., Haase P., Fohrer N. & J€ahnig S.C. (2015b) An attack on two fronts: predicting how changes in land use and climate affect the distribution of stream macroinvertebrates. Freshwater Biology, 60, 1443–1458. Kujala H., Moilanen A., Ara ujo M.B. & Cabeza M. (2013) Conservation planning with uncertain climate change projections. PLoS One, 8, e53315. Leigh C., Bush A., Harrison E.T., Ho S.S., Luke L., Rolls R.J. et al. (2014) Ecological effects of extreme climatic events on riverine ecosystems: insights from Australia. Freshwater Biology, 60, 2620–2638. Liu C., White M. & Newell G. (2013) Selecting thresholds for the prediction of species occurrence with presenceonly data. Journal of Biogeography, 40, 778–789. Lois S., Cowley D.E., Outeiro A., San Miguel E., Amaro R. & Ondina P. (2014) Spatial extent of biotic interactions affects species distribution and abundance in river networks: the freshwater pearl mussel and its hosts. Journal of Biogeography, 42, 229–240. Lukasiewicz A., Finlayson C.M. & Pittock J. (2013) Identifying Low Risk Climate Change Adaptation in Catchment Management while Avoiding Unintended Consequences. National Climate Change Adaptation Research Facility, Gold Coast. Lukasiewicz A., Finlayson C.M. & Pittock J. (2014) Incorporating Climate Change Adaptation into Catchment Management: A User Guide. Report No. 76, Institute for Land, Water and Society, Charles Sturt University, Albury. Mackey B., Watson J.A.L. & Worboys G.L. (2010) Connectivity Conservation and the Great Eastern Ranges Corridor, An Independant Report of the Interstate Agency Working Group. Department for Environment, Climate Change and Water NSW, Sydney. Macneale K.H., Peckarsky B.L. & Likens G.E. (2005) Stable isotopes identify dispersal patterns of stonefly © 2016 John Wiley & Sons Ltd, Freshwater Biology, doi: 10.1111/fwb.12874 14 A. Bush & A. J. Hoskins populations living along stream corridors. Freshwater Biology, 50, 1117–1130. Maggini R., Kujala H., Taylor M., Lee J., Possingham H., Wintle B. et al. (2013) Protecting and Restoring Habitat to Help Australia’s Threatened Species Adapt to Climate Change. National Climate Change Adaptation Research Facility, Gold Coast. Mccormack R.B. (2012) A Guide to Australia’s Spiny Freshwater Crayfish. CSIRO Publishing, Canberra. Morrongiello J.R., Beatty S.J., Bennett J.C., Crook D.A., Ikedife D.N.E.N., Kennard M.J. et al. (2011) Climate change and its implications for Australia’s freshwater fish. Marine and Freshwater Research, 62, 1082–1098. Nakagawa S. & Schielzeth H. (2013) A general and simple method for obtaining R2 from generalized linear mixedeffects models. Methods in Ecology and Evolution, 4, 133– 142. Ormerod S.J. (2009) Climate change, river conservation and the adaptation challenge. Aquatic Conservation: Marine and Freshwater Ecosystems, 19, 609–613. Peters G.P., Andrew R.M., Boden T., Canadell J.G., Cias P., Le Quere C. et al. (2013) The challenge to keep global warming below 2°C. Nature Climate Change, 3, 4–6. Phillips S.J., Williams P., Midgley G. & Archer A. (2008) Optimising dispersal corridors for the Cape Proteaceae using network flow. Ecological Applications, 18, 1200–1211. Porfirio L.L., Harris R.M.B., Lefroy E.C., Hugh S., Gould S.F., Lee G. et al. (2014) Improving the use of species distribution models in conservation planning and management under climate change. PLoS One, 9, e113749. Raadik T. (2013) Systematic Revision of the Mountain Galaxias, €nther, 1866 Species Complex (Teleostei: Galaxias olidus Gu Galaxiidae) in Eastern Australia. Division of Science and Design, University of Canberra, Canberra. Radinger J. & Wolter C. (2014) Patterns and predictors of fish dispersal in rivers. Fish and Fisheries, 15, 456–473. Reside A.E., Vanderwal J. & Kutt A.S. (2012) Projected changes in distributions of Australian tropical savanna birds under climate change using three dispersal scenarios. Ecology and Evolution, 2, 705–718. Robson B.J., Chester E.T., Allen M., Beatty S., Close P., Cook B. et al. (2013) Novel Methods for Managing Freshwater Refuges Against Climate Change in Southern Australia. National Climate Change Adaptation Research Facility, Gold Coast. Rogelj J., Meinshausen M. & Knutti R. (2012) Global warming under old and new scenarios using IPCC climate sensitivity range estimates. Nature Climate Change, 2, 248–253. Saupe E.E., Barve V., Myers C.E., Sober on J., Barve N., Hensz C.M. et al. (2012) Variation in niche and distribution model performance: the need for a priori assessment of key causal factors. Ecological Modelling, 237–238, 11–22. Simaika J.P., Samways M.J., Kipping J., Suhling F., Dijkstra K.-D.B., Clausnitzer V. et al. (2013) Continental-scale conservation prioritization of African dragonflies. Biological Conservation, 157, 245–254. Stein J. & Nevill J. (2011) Counting Australia’s protected rivers. Ecological Management & Restoration, 12, 200–206. Sternberg D. & Kennard M.J. (2013) Environmental, spatial and phylogenetic determinants of fish life-history traits and functional composition of Australian rivers. Freshwater Biology, 58, 1767–1778. Sutherland C., Fuller A.K. & Royle J.A. (2014) Modelling non-Euclidean movement and landscape connectivity in highly structured ecological networks. Methods in Ecology and Evolution, 6, 169–177. Synes N.W. & Osborne P.E. (2011) Choice of predictor variables as a source of uncertainty in continental-scale species distribution modelling under climate change. Global Ecology and Biogeography, 20, 904–914. Thuiller W., Lafourcade B., Engler R. & Ara ujo M.B. (2009) BIOMOD – a platform for ensemble forecasting of species distributions. Ecography, 32, 369–373. Tonkin J.D., Stoll S., Sundermann A. & Haase P. (2014) Dispersal distance and the pool of taxa, but not barriers, determine the colonisation of restored river reaches by benthic invertebrates. Freshwater Biology, 59, 1843–1855. Turak E., Marchant R., Barmuta L.A., Davis J., Choy S. & Metzeling L. (2011) River conservation in a changing world: invertebrate diversity and spatial prioritisation in south-eastern coastal Australia. Marine and Freshwater Research, 62, 300–311. Van Leeuwen C.H.A., Van Der Velde G., Van Groenendael J.M. & Klaassen M. (2012) Gut travellers: internal dispersal of aquatic organisms by waterfowl. Journal of Biogeography, 39, 2031–2040. Van Vuuren D.P., Edmonds J., Kainuma M., Riahi K., Thomson A., Hibbard K. et al. (2011) The representative concentration pathways: an overview. Climatic Change, 109, 5–31. Vanderwal J., Shoo L.P., Graham C. & Williams S.E. (2009) Selecting pseudo-absence data for presence-only distribution modeling: how far should you stray from what you know? Ecological Modelling, 220, 589–594. V€ or€ osmarty C.J., Mcintyre P.B., Gessner M.O., Dudgeon D., Prusevich A., Green P. et al. (2010) Global threats to human water security and river biodiversity. Nature, 467, 555–561. Warren D.L. & Seifert S.N. (2011) Ecological niche modeling in Maxent: the importance of model complexity and the performance of model selection criteria. Ecological Applications, 21, 335–342. Warren R., Price J., Fischlin A., Nava Santos S. & Midgley G. (2011) Increasing impacts of climate change upon ecosystems with increasing global mean temperature rise. Climatic Change, 106, 141–177. Watling J. & Braga L. (2015) Desiccation resistance explains amphibian distributions in a fragmented tropical forest landscape. Landscape Ecology, 30, 1449–1459. © 2016 John Wiley & Sons Ltd, Freshwater Biology, doi: 10.1111/fwb.12874 Dispersal ability and projected impacts of climate change Wiens J.A., Stralberg D., Jongsomjit D., Howell C.A. & Snyder M.A. (2009) Niches, models, and climate change: assessing the assumptions and uncertainties. Proceedings of the National Academy of Sciences of the United States of America, 106, 19729–19736. Williams P., Hannah L.E.E., Andelman S., Midgley G.U.Y., Ara ujo M., Hughes G. et al. (2005) Planning for climate change: identifying minimum-dispersal corridors for the Cape Proteaceae. Conservation Biology, 19, 1063– 1074. Wood S. & Scheipl F. (2014) gamm4: Generalized Additive Mixed Models Using mgcv and lme4. R package version 0.23. http://CRAN.R-project.org/package=gamm4. Wood S.N. (2004) Stable and efficient multiple smoothing parameter estimation for generalized additive models. Journal of the American Statistical Association, 99, 673–686. Wright A.N., Hijmans R.J., Schwartz M.W. & Shaffer H.B. (2015) Multiple sources of uncertainty affect metrics for 15 ranking conservation risk under climate change. Diversity and Distributions, 21, 111–122. Supporting Information Additional Supporting Information may be found in the online version of this article: Appendix S1. Sources and temporal distribution of species data. Appendix S2. Selected variable for all freshwater SDMs. Appendix S3. Model validation scores for all SDM algorithms and ensemble models. Appendix S4. R-code for calculating projected range shifts of taxa with different dispersal pathways. Appendix S5. Summary of generalised additive mixedeffects models. (Manuscript accepted 27 October 2016) © 2016 John Wiley & Sons Ltd, Freshwater Biology, doi: 10.1111/fwb.12874