* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download BIOTRANSFORMATION OF DRUGS
Enzyme inhibitor wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Metabolomics wikipedia , lookup
Citric acid cycle wikipedia , lookup
Drug design wikipedia , lookup
Biosynthesis wikipedia , lookup
Biochemistry wikipedia , lookup
Butyric acid wikipedia , lookup
Metalloprotein wikipedia , lookup
Drug discovery wikipedia , lookup
Epoxyeicosatrienoic acid wikipedia , lookup
15-Hydroxyeicosatetraenoic acid wikipedia , lookup
Specialized pro-resolving mediators wikipedia , lookup
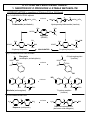
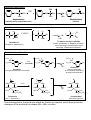
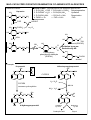
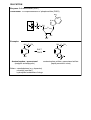
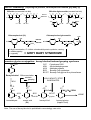
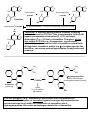
BIOTRANSFORMATION OF DRUGS Contents PART A: Biotransformation reactions Introduction Phase I reactions Phase II reacions The effect of biotransformation on the biological activity of drugs: How the biological (pharmacological and toxic) activity of the metabolite compares to that of the parent compound? PART B: Alterations in biotransformation Biological alterations - Decreased biotransformation capacity in neonates - Genetic alterations in biotransformation Mutation or multiplication of genes coding for drug metabolizing enzymes – possible consequences Chemical alterations - Induction of CYP – possible consequences - Inhibition of CYP – possible consequences INTRODUCTION ELIMINATION MECHANISMS Physical mechanism: Chemical mechanism: EXCRETION BIOTRANSFORMATION CONTRIBUTION OF EXCRETION AND BIOTRANSFORMATION TO ELIMINATION OF DRUGS - examples Drugs NOT biotransformed, excreted unchanged Drugs excreted both unchanged and as metabolites Drugs fully biotransformed, excreted only as metabolites Benzylpenicilin Aminoglycosides Metformin Tubocurarine Amantadine Salicylates Acetaminophen Phenobarbital TCADs Phenothiazines Chloramphenicol The sites of biotransformation: Predominantly, the liver The liver contributes to both the presystemic and the systemic elimination of many drugs. Often other tissues, as well. e.g., in intestinal mucosa cells presystemic elimination of several drugs in renal tubular cells, etc The colon, by bacteria - e.g., azo reduction, hydrolytic reactions BIOTRANSFORMATION: classification Phase II reactions (conjugations) Phase I reactions 1. Glucuronidation 2. Sulfation 3. Conjugation with glycine (Gly) 4. Conjugation with glutathione (GSH) 1. Oxidation 2. Reduction 5. Acetylation 6. Methylation 3. Hydrolysis The chemical role of Phase I and Phase II biotransformations: A functional group is added to the molecule or explored in the molecule at which conjugation can take place An organic acid (or acetyl or methyl group) is conjugated to the molecule at a preexisting functional group or at a functional group acquired in Phase I biotransformation General examples RH R-HC=O R-O-glucuronic acid oxidation O reduction 2H R1-O-(C=O)-R2 R-OH R-O-sulfonic acid R-CH2-O-glucuronic acid R-CH2-OH hydrolysis HOH R-CH2-O-sulfonic acid R-O-glucuronic acid R1-OH R-O-sulfonic acid The pharmacological role of Phase I and Phase II biotransformations: How the fate and effect of metabolites compare to the fate and effect of the parent drug? Phase I metabolites Phase II metabolites (conjugates) Water-solubility: (slightly faster excretion) Water-solubility: for 1-4: (rapid excr.) for 5-6: (slower excretion) Biological activity: in general but often Biological activity: almost always very seldom Part A. BIOTRANSFORMATION REACTIONS 1. PHASE I BIOTRANSFORMATIONS 1.1. OXIDATION 1.1.1. Microsomal oxidation 1.1.1.1. CYP-catalyzed reactions 1.1.1.1.1. Oxigenation 1.1.1.1.1.1. Insertion of oxygen produces a stable oxigenated metabolite 1.1.1.1.1.1.1. C-hydroxylation: aliphatic hydroxylation, aromatic hydroxylation 1.1.1.1.1.1.2. N-hydroxylation 1.1.1.1.1.1.3. Epoxidation 1.1.1.1.1.2. Insertion of oxygen produces an unstable oxigenated metabolite which undergoes spontaneous cleavage into two molecules 1.1.1.1.1.2.1. Oxidative dealkylation (N- and O-dealkylation) 1.1.1.1.1.2.2. Oxidative deamination 1.1.1.1.1.2.3. Oxidative dehalogenation 1.1.1.1.1.2.4. Oxidative desulfuration 1.1.1.1.2. Dehydrogenation 1.1.1.1.3. Reduction (reductive dehalogenation) 1.1.1.2. FMO-catalyzed oxidations 1.1.2. Non-microsomal oxidation 1.1.2.1. MAO-catalyzed oxidations 1.1.2.2. Oxidations catalyzed by molydenum-containing oxidases 1.1.2.2.1. Xanthine oxidase-catalyzed oxidations 1.1.2.2.2. Aldehyde oxidase-catalyzed oxidations 1.1.2.3. Alcohol dehydrogenase and aldehyde dehydrogenase-catalyzed oxidations 1.2. REDUCTION 1.2.1. Azo-reduction 1.2.2. Nitro-reduction 1.2.3. Carbonyl-, aldehyde- and aldose-reduction (by aldo-keto reductases; AKR) 1.3. HYDROLYSIS 1.3.1. Hydrolysis by carboxylesterases 1.3.2. Hydrolysis by alkaline phosphatase 1.3.3. Hydrolysis by paraoxonases 1.3.4. Hydrolysis by epoxide hydrolase (illustrated under epoxidation) 1.3.5. Hydrolysis by microbial hydrolases in the colon 2. PHASE II BIOTRANSFORMATIONS (CONJUGATIONS) 2.1. GLUCURONIDATION 2.2. SULFATION 2.3. CONJUGATION WITH GLYCINE 2.4. CONJUGATION WITH GLUTATHIONE (Glu-Cys-Gly) 2.5. ACETYLATION 2.6. METHYLATION PHASE I BIOTRANSFORMATIONS OXIDATION - summary MICROSOMAL NON-MICROSOMAL catalyzed by: catalyzed by: Cytochrome P-450 (CYP) Monoamino-oxidases (MAO) - mitochondrial Mo-containing oxidases (cytosolic): Microsomal flavine-containing monooxigenase (FMO) Xanthine oxidase (XO) and Aldehyde oxidase (AOX) Alcohol- and aldehyde dehydrogenases (ADH, ALDH) - cytosolic OXIDATIONS CATALYZED BY CYTOCHROME P-450 (CYP) CYP is a heme-containing protein embedded in the membranes of the smooth endoplasmic reticulum (SER), the fragments of which in a tissue homogenate are sedimented after ultracentrifugation (at 100,000g) in the microsomal fraction. Origin of the name cytochrome P-450: Cytochrome: it is a coloured intracellular protein P: it is pink 450: its absorption spectrum has a maximum at 450 nm CYP constitutes a superfamily of enzymes that are classified into families (numbered) and subfamilies (marked with capital letters), the latter of which contain the individual enzymes (numbered), e.g., CYP1A2, CYP2C9, CYP2D6, CYP3A4. CYTOCHROME P-450 (CYP)-CATALYZED REACTIONS Mechanisms CYP is an extremely versatile enzyme as it can catalyze numerous types of reaction, out of which three types are presented below. Oxygenation involves insertion of an O atom (from O2) into a C-H bond, forming a hydroxylated metabolite, or into a C=C double bond, forming an epoxide. A hydroxylated metabolite may be stable, or unstable. From an unstable hydroxylated metabolite a group may break off spontaneously: an alkyl group, ammonia, a halogen atom, or sulfur atom; such reactions are called oxidative dealkylation, oxidative deamination, oxidative dehalogenation, and oxidative desulfuration, respectively. CYP can also catalyze dehydrogenation, i.e. removal of 2 H atoms from a drug molecule (this is how the reactive hepatotoxic paracetamol metabolite is formed). Surprisingly, CYP may also catalyze reduction, by transferring only 1 electron to a compound (e.g. in a reductive dehalogenation reaction) or as many as 6 electrons to a nitro group, thus converting it into an amino group (nitro reduction). As catalyzed by CYP, these reductions are also shown here. I. OXYGENATION - the most typical CYP-catalyzed reaction General scheme: RH + O2 + (NADPH + H)+ CYP NADPH-CYP reductase R-OH + HOH + NADP+ Note: 1 atom of O2 is inserted into the drug, whereas the 2nd O atom is reduced to HOH by NADPH-CYP red. Catalytic cycle (simplified): 1 Product (ROH) Fe 3+ Fe ROH 7 6 (FeO)3+ RH HOH H + 2+ Fe OOH RH Substrate (RH) 3+ In the course of oxygenation by CYP, O2 is activated by electron uptake to form reactive O atoms. The first reactive form is the hydroperoxy form (produced in step 5). From this, 1 O is inserted into a water molecule (HOH). The second reactive form is the oxene (produced in step 6). From this, the reactive O atom is inserted into a C-H bond of the drug, forming a hydroxylated metabolite in step 7, which is then released from CYP. CYP-catalyzed dehydrogenation and reduction are explained below. 5 H Note that Fe represents the iron in CYP. e + _ 3+ Fe RH 2 e _ 2+ Fe RH 3 O2 3+ _ Fe O2 RH 4 NADPH-cytochrome P450 reductase II. DEHYDROGENATION e.g. acetaminophen (=paracetamol), nifedipine, ethanol 6 (FeO)3+ RH2 Fe R NOTE: The 2nd O atom is also reduced to water, using 2 H atoms from the drug molecule. 3+ 7 HOH III. REDUCTION e.g. nitro reduction of clonazepam; reductive dehalogenation of CCl4 2+ 4 Fe O2 RH 2+ e _ Fe O _2 [RH] 5 NOTE: The second electron is not transferred to the CYP-bound oxygen, but to the drug molecule. CYP-CATALYZED REACTIONS Overview OXYGENATION (insertion of one O atom) - two variations: 1. INSERTION OF O PRODUCES A STABLE METABOLITE: a. Hydroxylation C-hydroxylation (insertion of O into a C-H bond to form a hydroxyl group): - Aliphatic hydroxylation: - Aromatic hydroxylation: tolbutamide (-), terfenadine (+), cyclophosphamide (+) warfarin (-), phenytoin (-), propranolol (-) N-hydroxylation (insertion of O into an N-H bond): dapsone (†) b. Epoxidation (insertion of O into a C=C bond to form an epoxide): carbamazepine (-/o) 2. INSERTION OF O PRODUCES AN UNSTABLE METABOLITE WHICH SPONTANEOUSLY BRAKES INTO TWO MOLECULES: a. Oxidative dealkylation ( dealkylated metabolite + an aldehyde): N-dealkylation: Diazepam nordiazepam (+), Carisoprodol meprobamate (+), Amitriptyline nortriptyline (+) desmethyl-nortriptyline (-) Lidocaine monoethylglycylxylidine (+) glycylxylidine (-) Caffeine paraxanthine (-), or theophylline, or theobromine O-dealkylation: Codeine morphine (+) Dextromethorphan dextrorphan (=D form of levorphanol) (+) Phenacetin acetaminophen (=paracetamol) (+) b. Oxidative deamination ( O-containing metabolite + NH3): Amphetamine phenylacetone (-) c. Oxidative dehalogenation ( O-containing metabolite + HBr): Halothane trifluoroacetyl chloride (†) d. Oxidative desulfuration ( O-containing metabolite + S): Thiopenthal pentobarbital (+), Parathion paraoxon (+); thio-TEPA TEPA (+) DEHYDROGENATION (removal of 2 H atoms): Acetaminophen N-acetylbenzoquinone imine (†) Nifedipine (a dihydropyridine parent compund) ”pyridine” metabolite (-) REDUCTION (reductive dehalogenation, nitro reduction): CCl4 CCl3 (†) + Cl¯; Halothane debrominated halothane free radical (†) + Br¯ For reduction of nitro group of clonazepam (or nitrazepam) to amino group by CYP, see under nitro reduction (6-electron reduction) + = active metabolite; = inactive metabolite; † = toxic metabolite CYP-CATALYZED REACTIONS: 1. NSERTION OF O PRODUCES A STABLE METABOLITE HYDROXYLATION = insertion of O into a C-H or N-H bond a) C-HYDROXYLATION: aliphatic O O S NH C NH O CH3 CYP2C9 Tolbutamide (antidiabetic) Hydroxymethyl-tolbutamide (inactive) CH3 CH3 HOOC CH2 CH CH CH3 Ibuprofen (NSAID) CH3 CH3 HOOC CH O O S NH C NH O HO CH2 CH2 OH CH3 HOOC CH2 C OH CH CH2 CH CH3 CH3 3-hydroxy-ibuprofen CONJUGATES 2-hydroxy-ibuprofen b) C-HYDROXYLATION: aromatic Phenytoin (antieileptic, antiarrhythmic) O HN HN NH C6H5 4'-Hydroxyphenytoin (inactive) O CYP2C9 NH C6H5 O O OH O O OH CYP2C9 HO O O H H C C CH2 C Warfarin (anticoagulant) CH3 OH CH2 C 7-hydroxy-warfarin (inactive) O CH3 O c) N-HYDROXYLATION O H2 N S O Dapsone (antileprotic) NH2 CYP2C9 O H2 N S O Dapsone-hydroxylamine (hemotoxic and allergenic) NH OH EPOXIDATION = insertion of O into a C=C double bond H O H CYP N N C O NH2 Carbamazepine-10,11-epoxide (less active metabolite) C O NH2 Carbamazepine (antiepileptic) Epoxide hydrolase CYP O CYP (+) (+) (+) O (+) HO HO OH benzo(a)pyrene benzo(a)pyrene-7,8-oxide benzo(a)pyrene-7,8-dihydro-diol OH benzo(a)pyrene7,8-dihydro-diol-9,10-epoxide "ultimate carcinogen" NOTE: Leucotriene A4 (LTA4) is also an epoxide! LTA4 is converted into either LTB4 (a diol) by epoxide hydrolase, or into LTC4 (a GSH conjugate) by glutathione S-transferase. CYP-CATALYZED REACTIONS: 2. INSERTION OF O PRODUCES AN UNSTABLE METABOLITE WHICH UNDERGOES SPONTANEOUS CLEAVAGE INTO TWO MOLECULES First, the drug becomes hydroxylated at the C atom of the alkyl group that is linked to the N (or the O) atom. This hydroxylated metabolite is unstable. It breaks spontaneously into two molecules: the dealkylated metabolite (e.g., an amine or alcohol/phenol), and an aldehyde (e.g., formaldehyde after demethylation, acetaldehyde after deethylation, etc.). N-dealkylation General scheme: NOTE: After hydroxylation at the R NH2 N-bound C atom of the alkyl group, the alkyl group breaks off (as an aldehyde) O from the N atom, producing a dealkylated amine metabolite H C H of the drug molecule. OH R NH CH2 R NH CH3 Examples: CH3 H O N O N CYP2C19 CYP3A4 Cl N HCHO Diazepam Cl N Other examples: lidocaine imipramine erythromycine monoethylglycylxylidine desmethylimipramine desmethylerythromycine Nordiazepam Theophylline may undergo: - N-demethylation at N1 and N3 by CYP - C-hydroxylation at C8 by CYP - N- methylation at N7 by MT O N3-demethylation H3C N N CYP1A2 O H N N H 1-Methylxanthine (25 %) O H3C O CH3 N N1 7 3 N N O H3C MT SAM O H N N N CH3 CH3 Caffeine Theophylline N In neonates more caffeine is formed from theophylline than in adults because the CYP levels in the neonatal liver are low, however, the methyltransferase (MT) levels are relatively high. O H3C Hydroxylation CYP3A4, CYP2E1 H O N N OH O N N CH3 O N1-demethylation CYP1A2 O CH3 N N N CYP1A2 H N N OH O N N H 1,3-Dimethyluric acid (50 %) H N3-demethylation H3C N CH3 3-Methylxanthine (15 %) 1-Methyluric acid (5 %) O-dealkylation General scheme: R O CH3 NOTE: After hydroxylation at the R OH O-bound C atom of the alkyl group, the alkyl group breaks off (as an aldehyde) O from the O atom, producing a dealkylated hydroxylated metabolite H C of the drug molecule. H OH R O CH2 Example: Other examples: codeine phenacetine HO CH3 O CYP2D6 HCHO N CH3 Dextromethorphan (antitussive drug) N CH3 Dextrorphan (active metabolite) morphine paracetamol Oxidative deamination CYP CH2 CH CH3 OH O CH2 C CH3 CH2 C CH3 NH2 NH2 NH3 Amphetamine (centrally acting sympathomimetic) Phenylacetone (inactive) Oxidative dehalogenation F F Cl C C F H F Br C YP2E1 F Cl (+) F C C F OH F Br C HBr Cl C O F T rifluoro-acetyl-chloride binds covalently to hepatic proteins, thus form ing a neoantigene and causing "halothane hepatitis" Halothane (inhalation anesthetic) Oxidative desulfuration S C2H 5 O P C 2H 5O O NO 2 O P CYP C 2H5 O P NO 2 O C 2H 5O [S] Parathion (an organophosphate insecticide) S S HN O H5 C2 NH N CYP O CH CH3 CH2 CH2 CH3 Thiopental (an i.v. anesthetic) H5 C 2 NO 2 O HN NH N O Paraoxon (active insecticide, an irreversible inhibitor of acethylcholinesterase) O HN O O C 2H 5 O S C 2H 5O O CH CH 3 CH 2CH 2CH3 [S] O NH N H5 C 2 O CH CH3 CH 2 CH 2 CH 3 Pentobarbital (active as hypnotic) The arrows point to the bond into which an O atom is inserted, which then promotes cleavage of the molecule to release NH3, HBr, or sulfur. CYP-CATALYZED REACTIONS: 3. DEHYDROGENATION General scheme: 6 (FeO)3+ RH2 Fe R 3+ 7 HOH Examples: H N O C CH3 N O C CH3 CYP1A2 CYP3A4 Acetaminophen analgetic and antipyretic drug NO2 O C OCH3 O H3CO C CYP2E1 OH NOTE: While an oxygenation produces 1 molecule hydroxylated metabolite plus 1 molecule HOH, dehydrogenation produces 2 molecules HOH. plus a dehydrogenated metabolite. NO2 O C OCH3 O H3CO C CYP3A4 O H3C CH3 N N-Acetyl-p-benzoH quinoneimine (NAPBQI) Nifedipine hepatotoxic a dihydropyridine Ca++-channel blocker H3C N CH3 "Pyridine metabolite" inactive Other example: Dehydrogenation of nicotine to nicotine 1’(5’) iminium ion (see under aldehyde oxidase) CYP-CATALYZED REACTIONS: 4. REDUCTION In CYP-catalyzed reduction, the second electron is not transferred to the CYP-bound O2, but to the CYP-bound substrate, e.g. carbon tetrachloride or clonazepam (see also under nitro reduction). With carbon tetrachloride, its reduced intermedier then undergoes a homolytic cleavage to form a free radical and a chloride anion. The reactive trichloromethyl free radical formed in the liver, can initiate lipid peroxidation in the liver cell membranes and can thus induce hepatic necrosis. General scheme: 2+ 4 2+ Fe O2 RH e Fe O _2 [RH] _ 5 Examples: 1. Reductive dehalogenation of carbon tetrachloride (1-electron reduction): Carbon tetrachloride Cl solvent Cl C Cl Cl CYP2E1 Cl reduction _ Cl- Cl Cl C : Cl Cl Cl Cl C. Tricloromethyl free radical initiates lipid peroxidation and liver injury 2. Nitro-reduction of clonazepam to 7-amino-clonazepam (6-electron reduction): H N H N O O CYP3A4 O2N CYP2C19 N Clonazepam anxiolytic and antiepileptic drug N Cl Cl 2e- R-NO HOH 7-aminoclonazepam inactive 2e- 2e- R-NO2 2H+ H2N R-NH2 R-NH-OH 2H+ 2H+ HOH NOTE for those interested: Homolytic cleavage occurs when a covalent bond (formed by two electrons shown as :) is cleaved in a way so that one electron remains with one of the breakage products, whereas the other electron will belong to the other breakage product. Thus, homolytic cleavage yields products with an unpaired electron (called free radicals), which are reactive. OXIDATIONS CATALYZED BY MICROSOMAL FLAVIN-CONTAINING MONOOXYGENASE (FMO) FMO-CATALYZED OXIDATIONS Overview Take place on heteroatoms in drugs: Oxidation of S atom: Cimetidine → cimetidine-S-oxide Oxidation of tert. N atom: Nicotine → nicotine-1’-N-oxide Trimethylamine → trimethylamine N-oxide (Deficiency: Fish-odor syndrome) OXIDATIONS CATALYZED BY FMO - Examples: O N-CN CH2 S CH2 CH2 NH C N NH CH3 N-CN CH2 S CH2 CH2 NH C N NH CH3 FMO N H CH3 N H Cimetidine (an H2-receptor antagonist) CH3 Cimetidine S-oxide (inactive) FMO N N Nicotine-1'-N-oxide (inactive) Nicotine CH3 CH3 N CH3 Trimethylamine endogenous volatile compound; accumulates in FMO3 deficiency, causing fish odor syndrome CH3 N CH3 N O FMO3 CH3 CH3 N O CH3 Trimethylamine N-oxide (water-soluble metabolite excreted in urine) OXIDATIONS CATALYZED BY NON-MICROSOMAL ENZYMES NON-MICROSOMAL OXIDATIONS Overview The oxygen incorporated into the substrate is derived from HOH rather than O2. OXIDATION CATALYZED BY MAO MAO catalyzes oxidative deamination of amines. Examples: - Dopamine → 3,4-dihydroxy-phenylacetaldehyde - Norepinephrine → 3,4-dihydroxy-mandelic aldehyde (-) - N-deisopropyl-propranolol → the respective aldehyde (-) - MPTP → MPP+(†) – kills the DAergic neurons, causing Parkinson’s disease OXIDATIONS CATALYZED BY MOLYBDENUM-CONTAINING OXIDASES: XANTHINE OXIDASE-CATALYZED OXIDATION: Examples: Hypoxanthine → xanthine → uric acid Allopurinol → alloxanthine (compet. inhibition of uric acid formation) 6-Mercaptopurine → 6-thiouric acid (-) ALDEHYDE OXIDASE-CATALYZED OXIDATION: Examples: - Nicotine 1’(5’) iminium ion → cotinine - Benzaldehyde → benzoic acid - 3-Methoxy 4-hydroxy-mandelic aldehyde → VMA OXIDATIONS CATALYZED BY ALCOHOL- AND ALDEHYDE DEHYDROGENASES: Examples: - Methanol → formaldehyde → formic acid (†) – causes blindness - Ethanol → acetaldehyde (†) → acetic acid - Ethylene glycol →→ glycolic acid (†) → glyoxylic acid → oxalic acid (†) – cause renal injury - Chloral (= trichloroacetaldehyde) → trichloroethanol (+) – ADH in the reverse reaction (using NADH) can reduce chloral; ethanol facilitates this by increasing NADH supply. MAO-CATALYZED OXIDATIVE DEAMINATION OF AMINES INTO ALDEHYDES General scheme: Dopamine HO HO CH2 CH2 NH2 FAD 1 FOUR STEPS - the byproducts are ammonia and HOOH! 1. R-CH2NH2 + FAD > R-CH=NH + FADH2 Dehydrogenation 2. R-CH=NH + H2O > R-CH(OH)-NH2 Hydration 3. R-CH(OH)-NH2 > R-CH=O + NH3 Deamination 4. FADH2 + O2 > FAD + H2O2 FAD regeneration HOOH ! 4 FADH2 HO HO O2 CH2 CH NH HO O HOH CH2 C HO 2 HO NH3 HO CH2 CH NH2 3 OH OH acid (DOPAC) HO O CH2 C HO H Oxidation by ALDH Reduction by AR HO 3,4-dihydroxyphenylacetyl aldehyde (DOPAL) CH2 CH2OH HO alcohol Example: Propranolol OH N-Desisopropylpropranolol OH CH3 O CH2 CH CH2 NH2 O CH2 CH CH2 NH CH CH3 CYP2D6 N-dealkylation O2 + H2O CYP2D6 MAO-A aromatic hydroxylation NH3 + H2O2 OH CH3 O CH2 CH CH2 NH CH OH CH3 OH 4-Hydroxypropranolol O O CH2 CH C H Oxidation by AOX Reduction by AR Carboxylic acid Glycol OXIDATIONS CATALYZED BY Mo-CONTAINING OXIDASES, XANTHINE OXIDASE (XO) and ALDEHYDE OXIDASE (AOX) Mechanism of oxygenation by XO and AOX – two steps: NOTE: The oxygen atom incorporated into the substrate is derived from water rather than O2. (1) A water molecule is added into the parent compound, forming an intermediary metabolite. (2) Thereafter, 2 H atoms are removed from the intermediary metabolite by molecular oxygen (O2), thus forming hydrogen peroxide (HOOH) and the oxygenated metabolite, which carries now a ketogroup or a carboxylic acid group. These 2 steps are shown only in the reactions presented for AOX. For simplicity, they are not shown in the reactions presented for XO. Oxygenations catalyzed by XO and/or AOX: 1. Oxygenation of a C atom double-bonded to a N atom in a heterocyclic ring, such as: in purines, e.g. hypoxanthine and xanthine (preferred by XO), in purine analogues, e.g. 6-mercaptopurine and 6-thioxanthine (preferred by XO), in zaleplone (preferred by AOX) in the dehydrogenated nicotine metabolite, nicotine-1’(5’)-iminium ion (mainly by AOX) 2. Oxygenation of aromatic (but not aliphatic) aldehydes, such as benzaldehyde (product of benzyl alcohol oxidation by ADH – see gasping syndrome). XANTHINE OXIDASE-CATALYZED OXIDATIONS O N HN N O O Hypoxanthine HN XO N H N O O HN XO O O N H N H HN N H N H Uric acid Xanthine N H N N H N Allopurinol to decrease hyperuricemia N HN N S S S N H 6-Mercaptopurine antitumor drug XO HN N N N H H 6-Thioxanthine O XO HN H N O O N N H H 6-Thiouric acid inactive Administration of the XO inhibitor allopurinol to patients treated with 6-mercaptopurine (6MP) would decrease the elimination of 6-mercaptopurine and in turn could result in 6MP-induced toxicity, i.e. bone marrow depression. Therefore, allopurinol is contraindicated for the 6-mercaptopurinetreated patient even if hyperuricemia developed. Patients have died because this contraindication was disregarded by ignorant physicians! NOTE: 6MP can also be eliminated by methylation at the SH group by thiopurine methyltransferase (TPMT, see later). TPMT deficiency increases the toxicity of 6MP, just like cotreatment with allopurinol does. ALDEHYDE OXIDASE-CATALYZED OXIDATIONS O O C CH3 C N CH3 C N CH2 CH3 AOX N HO N N H CN Zaleplon short-acting hypnotic CH3 N CH2 CH3 HOH N N O CH2 CH3 AOX O2 N HOOH N O CN N H N CN 5-oxo-zaleplon 5-hydroxy-zaleplon NOTE: Zaleplon is also N-deethylated by CYP3A4. CYP2A6 CYP2B6 N + N AOX AOX OH N N dehydrogenation N CH3 Nicotine CH3 N HOH CH3 N O2 HOOH nicotine-1'(5')-iminium ion O C H Benzaldehyde AOX HOH OH AOX OH C H O2 HOOH CH3 N cotinine O C OH Benzoic acid formed from benzylic alcohol, (see gasping syndrome) NOTE: Cotinine is the main metabolite of nicotine and as such it may be used as a biomarker of nicotine exposure (i.e. smoking). Cotinine is conveniently analyzed from the saliva of the smoker. O ALCOHOL DEHYDROGENASE (ADH)- AND ALDEHYDE DEHYDROGENASE (ALDH)-CATALYZED OXIDATIONS + NADH + H + NAD CH3 CH2 OH + CH3 OH CH2 OH CH2 OH H NADH + H O HOH H 3,4-dihydroxy-phenylacetyl aldehyde (DOPAL) formed by MAO in dopaminergic neurons acetic acid C NADH + H + O H C H ALDH OH formic acid Formic acid causes acidosis and retinal injury (blindness) H2C OH ALDH COOH glycol aldehyde Rate limiting step COOH HC O ADH ALDH COOH glyoxylic acid glycolic acid COOH oxalic acid Acidic metabolites cause acidosis and renal injury HO ALDH OH formaldehyde HC O HO CH2 C + OH H2C OH O ALDH NAD O CH3 C H OH H + NADH + H ADH NADH + H + DISULFIRAM to promote alcohol withdrawal H ETHANOL FOMEPIZOLE (antidotes) + NAD + NAD OH H C ADH Ethylene glycol ETHANOL FOMEPIZOLE (antidotes) HO CH3 C acetaldehyde NAD + OH CH3 C ADH Ethanol Methanol O HOH H3CO H3CO O O HO CH2 C HO OH 3,4-dihydroxyphenylacetic acid (DOPAC) CH OH O ALDH C HO CH H OH Vanillyl mandelic aldehyde formed by COMT and MAO from epinephrine and NE NOTES: 1. Fomepizole (=4-methylpyrazole; ANTIZOL®) is a potent competitive inhibitor of ADH and is the most reliable antidote in poisonings with methanol or ethylene glycol. Fomepizole is also used to alleviate the acetaldehyde syndrome caused by ethanol consumption in disulfiram-treated patients. C OH Vanillyl mandelic acid (VMA) CH3 N N H fomepizole 4-methylpyrazole ANTIZOL 2. As shown in Part 6 (Appendix-2), disulfiram is biotransformed into an electrophilic metabolite which covalently binds to and irreversibly inhibits aldehyde dehydrogenase. For this reason, a disulfiram-treated individual should not consume alcohol for at least a week after discontinuation of disulfiram administration. 3. Genetic and gender variations in the biotransformation of ethanol are presented in Part 6, Appendix-3. 4. Elimination of the potentially toxic DOPAL by ALDH from nigrostriatal dopaminergic neurons is a protective mechanism. Inhibition of ALDH by some pesticides (e.g. the fungicide benomyl) has been speculated to be a mechanism of pesticide-induced Parkinson disease. 5. VMA is the final product of the biotransformation of epinephrine (E) and norepinephrine (NE) via the COMT – MAO – ALDH pathway. Thus, VMA is the biomarker of E and NE production. Urinary excretion of large amounts of VMA is a diagnostic sign for pheochromocytoma. REDUCTION Overview AZO-REDUCTION (R1-N=N-R2 R1-NH2 + R2-NH2) catalyzed by microbial enzymes in the colon Prontosyl sulfanilamide + triaminobenzene Sulfasalazine (salicylazosulfapyridine) 5-aminosalycylic acid + sulfapyridine NITRO-REDUCTION (R-NO2 R-NH2): catalyzed by CYP Clonazepam 7-amino-clonazepam Chloramphenicol an arylamine metabolite (†) CARBONYL-, ALDEHYDE-, and ALDOSE-REDUCTION (R-C=O → R-C-OH): catalyzed by cytosolic enzymes of the aldo-keto reductase (AKR) superfamily, using NADPH Haloperidol reduced haloperidol Oxcarbazepin 10-hydroxy-carbazepin (+) Doxorubicin doxorubicinol (†) 3,4-Dihydroxyphenylacetaldehyde (DOPAL; produced from DA by MAO) 3,4-dihydroxyphenylethanol (DOPET) REDUCTION AZO-REDUCTION R N N R' R NH2 + R' NH2 O H2N N N O H2N S NH2 NH2 colonic bacteria O NH2 Prontosil S N H N N HO colonic HOOC bacteria N S NH2 NH2 (Gerhard Domagk, Nobel Prize, 1939) O H2N O tiraminobenzene HOOC + sulfanilamide (antibacterial, the prototype of sulfonamides) O NH2 HO + S N H H2N O O Sulfasalazine (Salicylazosulfapyridine) (to treat ulcerative colitis) NITRO-REDUCTION (R-NO2 Clonazepam (anxiolytic and antiepileptic effects) N R-NO H N sulfapyridine 5-aminosalycylic acid (antiinflammatory) R-NHOH R-NH2) H N O O CYP3A4 O2N CYP2C19 N H2N N R CH2 OH CARBONYL-REDUCTION R CH O O 7-aminoclonazepam (inactive) Cl Cl OH OH C CH2 CH2 CH2 N AKR OH CH CH2 CH2 CH2 N Cl Cl Haloperidol (antipsychotic) F Oxcarbazepine (antiepileptic, inactive prodrug) reduced haloperidol (inactive) F O OH AKR N O O 10-hydroxycarbazepine (active) C N O NH2 OH C O O C CH2OH NH2 OH OH CH CH2OH OH OH AKR OCH3 O Doxorubicin (antitumor drug) OH O O OCH3 O OH O CH3 CH3 HO HO NH2 AKR = aldo-keto reductase NH2 O doxorubicinol (cardiotoxic) HYDROLYSIS Overview BY CARBOXYLESTERASES (CES, microsomal enzymes) and/or BUTYRYLCHOLINESTERASES (BChE, soluble enzymes, also in plasma) HYDROLYSIS OF CARBOXYLIC ACID ESTERS: rapid Examples: - Drugs: Succinylcholine, mivacurium, procaine, tetracaine, cocaine, esmolol, meperidine, remifentanil, acetylsalicylic acid, clopidogrel, oseltamivir – the active drugs are converted into inactive (or less active) metabolites - Prodrugs: Fibrate esters, e.g. fenofibrate (-) fenofibric acid (+); ACE-inhibitor esters, e.g. enalapril (-) enalaprilat (+) Antipsychotic esters, e.g. pipothiazine palmitate (-) pipothiazine (+) Mycophenolate mofetil (-) mycophenolic acid (+) – by CES Bambuterol terbutaline (+); Also: heroin (+) morphine (+) HYDROLYSIS OF CARBOXYLIC ACID AMIDES: slow Example: Procainamide BY ALKALINE PHOSPHATASES: hydrolyzes phosphoric acid monoesters Examples (i.v. injectable prodrugs): Fosphenytoin (-) phenytoin (+) Fospropofol (-) propofol (+) Clindamycin phosphate (-) clindamycin (+) BY PARAOXONASES (Lactonases) HYDROLYSIS OF PHOSPHORIC ACID TRI-ESTERS Examples: Paraoxon and other organophosphate insecticides HYDROLYSIS OF LACTONES Examples: Statins, e.g. lovastatin, or simvastatin (lactone) hydroxy-acid (+), Spironolactone hydroxy-acid (-) BY EPOXIDE HYDROLASE: hydrolyzes the epoxide into dihydrodiols Examples: Carbamazepine-epoxide (little-reactive epoxide, active metabolite) Benzpyrene-epoxide (DNA-reactive epoxide, mutagenic and carcinogenic) NOTE: Leucotriene A4 (LTA4) is also an epoxide! It is converted into either LTB4 (a diol) by epoxide hydrolase, or into LTC4 (a GSH conjugate) by glutathione S-transferase. BY PANCREATIC LIPASE IN THE SMALL INTESTINE Example: castor oil (ricinoleic acid-containing triglyceride) ricinoleic acid (+) BY MICROBIAL HYDROLASES IN THE COLON Examples (laxative prodrugs): Sennoside A, bisacodyl, sodium picosulfate (-) = inactive metabolite, (+) = active metabolite, ASA = acetylsalicylic acid NOTE: BChE is protective against organic phosphate ester insecticides and “nerve agents” whose oxone form phosphorylates and irreversibly inactivates not only acetylcholinesterase (causing the toxicity) but also BChE, which has no role in the toxic action. Thus BChE represents a “silent binding site” for OP insecticides and chemical warfare agents by preventing their binding to the target of toxic action, i.e. the neuronal acetylcholinesterase. HYDROLYSIS BY CARBOXYLESTERASES and/or BUTYRYLCHOLINESTERASES esterase HOH General scheme: R C O R' O R COOH + HO R' DRUGS inactivated by hydrolysis: H3C O CH NH CH2 CH CH2 O H3 C CH2 CH2 C O CH3 OH + O CH3 Esmolol (short acting beta-antagonist) O CH3 CH2 C O CH2 CH2 N CH3 + CH3 CH2 C O CH2 CH2 N CH3 CH3 O C O N CH2 CH2 C O CH3 Succinylcholine O CH3 CH2 C N O CH3 (short acting muscle relaxant) Remifentanil (short acting opioid) CH2 CH3 O C H H2N O CH3 Clopidogrel (inhibitor of platelet aggregation) N Cl C O CH2 CH2 N O CH2 CH3 Procaine (short acting local anesthetic; rapidly hydrolyzed) S O COOH O C CH2 CH3 H2N CH3 C NH CH2 CH2 N O Acetylsalicylic acid (aspirin) CH2 CH3 Procainamide (antiarhytmic drug; slowly hydrolyzed) PRODRUGS activated by hydrolysis: O COOH C O C2H5 CH3 CH2 CH2 CH NH CH C N Enalapril Cl CH3 O C O CH3 C O CH CH3 CH3 O O C Fenofibrate (ACE inhibitor) (triglyceride-lowering drug) CH2 CH2 CH2 N CH2 CH2 O C CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH3 O O N CH3 S N O CH3 Pipothiazine palmitate (PIPORTIL a depot antipsychotic, injected i.m. as an oily injection once a month) S Some drugs (e.g., enalapril) containing carboxyl groups are esterified with an alcohol to make them more lipophilic for better absorption by diffusion from the GI tract. After absorption, such a drug is readiliy hydrolyzed by carboxylesterase and the original organic acid (e.g., enalaprilate from enalapril) is released. Enalapril is an inactiv drug (i.e., prodrug), whereas enalaprilate is the active ACE inhibitor metabolite. Similarly, the lipophilic heroin readily diffuses into brain, where its hydrolysis produces morphine. HYDROLYSIS BY ALKALINE PHOSPHATASE (AP) NOTE: Fosphenytoin, fospropofol and clindamycin phosphate are phosphate ester prodrugs that were synthesized in order to convert the poorly water-soluble phenytoin, propofol and clindamycin, respectively, into water-soluble, i.v.-injectable forms. As phenytoin does not contain a hydroxyl group at which to esterify it with phosphoric acid, and the hydroxyl group of propofol is sterically hindered from phosphoric acid by the neighboring isopropyl groups, first a -CH2-OH group had to be linked to these molecules. This linker group breaks off spontaneously as formaldehyde after the AP-catalyzed hydrolysis. HOH H N O N O OH HO OH O O O O CH2 OH P HCHO O Propofol OH N HOH Cl Cl AP N H N H OH O O SCH3 HO P OH O O HO SCH3 OH OH Clindamycin phosphate OH OH Phospropofol N O P O OH O spontaneous cleavage AP HO OH HCHO CH2 OH HOH OH HO O N H Phenytoin CH2 O P O O N OH Phosphenytoin OH P H N O OH CH2 O P O spontaneous cleavage H N AP Clindamycin OH HYDROLYSIS BY PARAOXONASES (PON) Hydrolysis of phosphoric acid tri-esters: O H3C CH2 O P H3C CH2 O O H3C CH2 O PON + P O NO2 H3C CH2 O HO NO2 OH Paraoxon (the active metabolite of parathion, an OP insecticide, irreversble acethylcholinesterase inhibitor) Hydrolysis of lactone rings: O HO O PON HO O OH OH C PON O CH3 O H3C O H3C CH3 O O active lovastatin H3C O H3C O C OH OH inactive spironolacton S C CH3 Lovastatin Spironolactone (HMG-CoA reductase inhibitor, cholesterol lowering drug) (aldosterone antagonist, K-sparing diuretic) Notes: - PON is also called aromatic esterase 1 or serum aryl-dialkylphosphatase 1 - PON1 is synthesized in the liver and transported along with HDL in the plasma. It functions as an antioxidant; it is an anti-atherosclerotic component of HDL. HYDROLYSIS BY MICROBIAL HYDROLASES IN THE COLON Hydrolysis produces stimulatory laxatives in the colon OH Sennoside A HO OH O O HO OH O OH O COOH COOH COOH 2 HOH COOH COOH 2 glucose COOH O O OH HO O HO OH Bisacodyl OH active antraquinone aglycone antraquinone glycoside (prodrug) OH O N N 2 HOH O O 2 acetate H3C O O HO CH3 Active diphenol-methane laxative metabolite Diacetic acid ester of diphenol-methane laxative (prodrug) Sodium picosulfate N N 2 HOH O O S NaO 2 sulfate S O O OH O ONa HO OH O Disulfonic acid ester of diphenol-methane laxative (prodrug) Active diphenol-methane laxative metabolite PHASE II BIOTRANSFORMATIONS (CONJUGATIONS) GLUCURONIDATION Enzyme Cosubstrate Substrates: drugs, endogenous compounds UDP-glucuronosyl transferases UDP-glucuronic OH-containing compounds: morphine, paracetamol, acid chloramphenicol, oxazepam, phenolphthalein, (in the endoplasmic propofol, diethylstilbestrol, estradiol, thyroxine reticulum = microsomes) OH-contaning metabolites: 6-OH phenytoin, etc. N-OH-dapsone COOH-containing compounds: valproic acid, diclophenac, furosemide, telmisartan, bilirubin COOH-containing metabolites: fenofibric acid N-containing drugs: lamotrigine SULFATION Enzyme Cosubstrate Substrates Sulfotransferases PAPS OH-containing compounds: pravastatin + see above OH-contaning metabolites: see above N-oxide-containing compounds: minoxidil (in cytosol) CONJUGATION WITH GLYCINE Enzyme Cosubstrate Acyl-CoA synthetase ATP + Glycine N-acyltransf. + CoA-SH (in mitochondrial matrix) + Glycine Substrates COOH-containing compounds: salicylic cid, benzoic acid, nicotinic acid CONJUGATION WITH GLUTATHIONE (Glu-Cys-Gly) Enzyme Cosubstrate Substrates Glutathione S-transf. Glutathione (glu-cys-gly) Electrophilic C-containing compounds: ethacrinic acid, epoxides (e.g. LTA4) Electrophilic C-containing metabolites: N-acetyl-p-benzoquinone imine, acrolein (in cytosol) ACETYLATION Enzyme Cosubstrate Substrates N-acetyltransferases (in cytosol) Acetyl-CoA NH2-containing compounds: NAT1 substrates: p-aminobenzoic acid, sulfamethoxazol NAT2 substrates: isoniazid, procainamide, dapsone, hydralazine, sulfamethazine, METHYLATION Enzyme Cosubstrate Substrates Methyltransferases S-adenosylmethionine OH-containing compounds: L-Dopa (COMT) N-containing compounds: histamine, nicotine SH-cont. compounds: 6-mercaptopurine (TPMT) (in cytosol) GLUCURONIDATION Enzymes: UDP-glucuronosyltransferases (UDP-GT, UGT) Cosubstrate: O UDP-glucuronic acid (UDP-GA) NH O O N _ COO O O CH2 O P O P O O O _ O _ HO OH OH OH OH Examples: - Ether glucuronide formation (from compounds with a hydroxyl group) COO _ O OH O2N OH UDP-GT CH CH CH2 OH NH UDP-GA O2N HO OH CH CH CH2 O C CHCl 2 C CHCl2 OH O NH O Chloramphenicol (antibiotic) Chloramphenicol glucuronide (rapidly excreted in urine) Others: - Endogenous compounds: Thyroxine, Estradiol - Drugs: Acetaminophen, Diethylstilbestrol, Ezetimibe, Morphine, Oxazepam, Phenolphthalein - Hydroxylated metabolites of drugs - Ester glucuronide formation (from compounds with a carboxylic group) CH3 CH2 CH2 O UDP-GT CH C CH3 CH2 CH2 COO CH3 CH2 CH2 CH UDP-GA O O C CH3 CH2 CH2 OH _ HO OH O OH Valproic acid (antiepileptic drug) Valproic acid glucuronide (rapidly excreted in urine) Others: - Bilirubin (forms mono- and diglucuronides) - Drugs: NSAID (e.g., diclofenac), furosemide, telmisartan - Acidic metabolites (e.g., fenofibric acid) produced from fibric acid esters by ester hydrolysis - N-glucuronide formation N H2N N N UDP-GT Lamotrigine (antiepileptic drug) Other: Carbamazepine NH2 N Cl Cl _ O + HO OH N UDP-GA Cl Cl N H2N NH2 COO OH Lamotrigine N2-glucuronide (rapidly excreted in urine) SULFATION Enzymes: Sulfotransferases (SULT) Cosubstrate: 3'-Phosphoadenosine-5'-phosphosulfate (PAPS) NH2 N N O O N N O S O P O CH2 O O O O OH PO32- Examples: NH C CH3 O NH C CH3 O SULT PAPS OH Acetaminophen = paracetamol (analgetic and antipyretic) Others: catecholamines (e.g., dopamine) minoxidyl (see later) hydroxylated metabolites of drugs O O S O O acetaminophen-sulfate = paracetamol-sulfate (rapidly excreted in urine) CONJUGATION WITH GLYCINE Enzymes: (1) acyl-CoA synthetase (ACS) (2) acyl-CoA: glycine N-acyltransferase (GNT) Both enzymes are located in the mitochondrial matrix. Cosubstrates: (1) ATP, (2) Coenzyme A (CoA-SH), (3) Glycine (Gly) Examples: C O C OH OH ACS O S CoA OH GNT Salicyl-CoA NH CH2 COOH OH Salicyl-glycine = salicyluric acid (rapidly excreted in urine) carboxylesterase acetylation and inactivation of COX Acetylsalicylic acid (aspirin) GNT ACS Benzoic acid O Gly ATP, CoA-SH Salicylic acid C ATP, CoA-SH Benzoyl-CoA Gly Benzoyl-glycine (hippuric acid) AOX Benzadehyde ADH Benzyl alcohol (an antiseptic; see under gasping syndrome) Note: Benzoyl-glycine was the first identified metabolite of a xenobiotic. In 1830, it was found in the urine of horses given benzoic acid. Then it was named hippuric acid (hippos means horse in Greek). CONJUGATION WITH GLUTATHIONE Enzymes: Glutathione S-transfrerases (GST) Cosubstrate: Glutathione (GSH) COO CH _ O O CH2 CH2 C C NH NH CH2 COO _ CH NH2 CH2 SH .. -glutamic acid cysteine glycine Notice that the SH group in GSH is electron-rich (i.e., nucleophilic), therefore it is reactive with electron-deficient (i.e., electrophilic) atoms, thus forming a glutathione conjugate. As drugs and chemicals with elecrophilic atoms may also bind to protein-cysteines, conjugation with glutathione prevents their covalent binding to proteins. Therefore, conjugation with GSH is an important protective mechanism against reactive electrophiles, such as NAPBQI. Examples: Compounds with electrophilic (electron deficient, partially positive) carbon atoms O CH2 COOH O CH2 COOH Cl Cl GST GSH Cl Cl O C C CH2 CH3 (+) CH O C CH CH2 CH3 CH2 SG 2 Ethacrynic acid (EA) EA-glutathione conjugate (loop diuretic) Note: the carbon with (+) sign is electron-deficient (contains a partial positive charge), thus it is electrophilic, therefore reactive with GSH, whose SH group is electron-rich (nucleophilic). O (+) Acrolein, a reactive electrophilic CH2 CH C H aldehyde that causes hemorrhagic cystitis in CP-treated patients EA-Cys conjugate (active diuretic metabolite) O Acrolein-glutathione conjugate GS CH2 CH2 C (detoxified) H GSH Cyclophosphamide (CP) (antitumor drug, a nitogen-mustard) HN C CH3 O N C CH3 O CYP2E1 toxication OH Acetaminophen = paracetamol HN C CH3 O GSH (+) (+) detoxication O N-acetyl-p-benzoquinoneimine (NAPBQI) ELECTROPHILIC METABOLITE MAY CAUSE HEPATIC NECROSIS SG OH Acetaminophen-glutathione conjugate (readily excreted) ACETYLATION Enzymes: N-acetyltransferases (NAT) Cosubstrate: Acetyl coenzyme A (Ac-CoA) NH2 N N O CH3 O O N N NH C CH C CH2 O P O P O CH2 OH CH3 CH2 O O O CH2 C O O O NH CH2 CH2 S C CH3 PO3H OH - Examples: O O C C NH NH2 O NH NH C CH3 NAT2 Ac-CoA N Isoniazid (antituberculotic) N Acetylisoniazid (inactive metabolite) Others: NAT1 substrates: p-Aaminobenzoic acid, p-Aminosalicylic acid, Sulfamethoxazole NAT2 substrates: Dapsone, Procainamide, Hydralazine, Sulfamethazine METHYLATION Enzymes: Methyltransferases (MT) Cosubstrate: S-adenosylmethionine (SAM) NH2 N N _ CH3 OOC CH CH2 CH2 S + NH2 N N CH2 O OH OH Examples: entacapone O-methylation CH3 O HO CH2 CH COO HO _ NH2 COMT SAM CH2 CH COO HO _ NH2 L-Dopa (antiparkinson drug, dopamine precursor) 3-O-Methyl-L-Dopa N-methylation CH2 CH2 NH2 CH2 CH2 NH2 HMT HN N SAM CH3 N N N-Methylhistamine Histamine S-methylation SH S CH3 N N N N H 6-Mercaptopurine (antitumor drug) TPMT N N SAM N N H 6-Methylmercaptopurine Note: In patients with thiopurine methyltransferase (TPMT) deficiency (like in patients treated with allopurinol – see earlier), 6-mercaptopurine elimination is slow. In such patients, this drug may not cause apoptosis of leukemic cells only (which is desirable), but may also induce apoptosis of normal bone marrow cells as well! EFFECT OF BIOTRANSFORM ATION ON BIOLOGICAL ACTIVITY 1. TYPICALLY: ACTIVE DRUG INACTIVE METABOLITE warfarin phenytoin theophylline morphine acetaminophen isoniazide 7-hydroxy-warfarin 4-hydroxy-phenytoin 1- or 3-methylxanthine morphine-3-glucuronide acetaminophen-glucuronide acetyl-isoniazide CYP2C9 CYP2C9 CYP2A1 UDP-GT UDP-GT NAT2 2. EXCEPTIONALLY: ACTIVE METABOLITE IS FORMED a. Inactive parent compound (PRODRUG) active metabolite cyclophosphamide tamoxifen parathion terfenadine chloral hydrate sulfasalazine oxcarbazepine lovastatin (lactone) enalapril (ester) fenofibrate (ester) ezetimibe minoxidyl ethacrynic acid (EA) phosphoramide mustard 4-hydroxy-tamoxifen paraoxon alcohol acid trichloroethanol 5-aminosalicylic acid 10-hydroxy-carbazepine lovastatin (free acid) enalaprilate (free acid) fenofibric acid (free acid) ezetimibe-glucuronide minoxidyl-sulfate EA-cysteine CYP2B6 CYP2D6 CYP3A4 CYP3A4 ADH (rev.), AR Azo-reductase AK-reductase Paraoxonase Esterase Esterase UDP-GT SULT GSTGGT, DP b. Active parent compound active metabolite phenylbutazone carisoprodol risperidone imipramine codeine diazepam morphine -OH-phenylbutazone meprobamate 9-OH-risperidone (paliperidone) desmethyl-imipramine morphine nordiazepam oxazepam morphine 6-glucuronide CYP2D6, 2C9 CYP2C19 CYP2D6 CYP2D6 CYP2D6 CYP CYP UDP-GT c. „Non-toxic” parent compound toxic metabolite acetaminophen halothane cyclophosphamide methanol ethylene glycol doxorubicin N-acetyl-p-benzoquinoneimine trifluoroacetyl chloride acrolein formic acid glycolic-, glyoxylic- oxalic acid doxorubicinol CYP2E1 CYP2E1 CYP3A4, 2B6 ADH ALDH ADH ALDH AK-reductase BIOTRANSFORMATION OF DIAZEPAM, FIRST INTO ACTIVE PHASE-I METABOLITES (NORDIAZEPAM, OXAZEPAM), AND FINALLY INTO INACTIVE OXAZEPAM-GLUCURONIDE PHASE-I biotransformation CH3 O N Cl Examples of drugs whose active metabolites are also drugs: Diazepam (antianxiety and antiepileptic drug) active [VALIUM] N T1/2= 40 hrs diazepam VALIUM > oxazepam SERAX risperidone RISPERDAL > paliperidon INVEGA terfenadine TELDANE* > fexofenadine TELFAST sulfasalazine SALAZOPYRIN > mesalazine SALOFALK enalapril ENALAPRIL tabl > enalaprilate VASOTEC inj * Withdrawn - see later N-demethylation CYP2C19 CYP3A4 H O N Cl Nordiazepam active metabolite N PHASE-II biotransformation (conjugation) Hydroxylation CYP H H O N O N UDP-GT OH Cl UDP-GA N O -Glucuronic Cl acid N T1/2= 8 hrs oxazepam active metabolite and also anti anxiety drug [SERAX] oxazepam-glucronide inactive and readily excreted metabolite HYDROXYLATION OF RISPERIDONE TO AN ACTIVE METABOLITE NOTE that both risperidone and its metabolite, paliperidone, are sold as drugs. F F OH H3C N H3C N CYP2D6 N N O N N N O O Risperidone (antipsychotic; RISPERDAL),T1/2 = 3 hrs N O 9-hydroxy-risperidone (active) paliperidone (INVEGA), T1/2 = 25 hrs BIOTRANSFORMATION OF CYCLOPHOSPHAMIDE INTO THERAPEUTICALLY ACTIVE METABOLITE (PHOSPHORAMIDE MUSTARD), SOME INACTIVE METABOLITES, AND A TOXIC METABOLITE (ACROLEIN) Cl O cyclophosphamide M= P (antitumor drug) N N H Cl O O GS N H O O P P N M O P M N H OH 4-glutathionylcyclophosphamide M CYP3A4 CYP2C9 CYP2B6 1 O imminocyclophosphamide O C H M H2N M detoxication O O ALDH1A1 P O 4-hydroxycyclophosphamide spontaneous cleavage 2 O aldophosphamide NOTE: Steps 1 and 2 represent N-dealkylation, starting with hydroxylation on the C atom linked to the N atom, then chain breaking at the arrow, and forming an aldehyde. Here the aldehyde is aldophosphamide. O C HO O spontaneous cleavage H C SG GSH detoxication H O O C HO P H2N M Acrolein reacts covalently with protein-SH Phosphoramide mustard reacts covalenly with DNA bases SPECIFIC TOXIC EFFECTS ANTITUMOR EFFECT and GENERAL TOXIC EFFECT of antitumor drugs e.g., bone marrow depression Liver: damage to the sinusoidal endothelial cells, causing sinusoidal obstruction sy. (SOS) Bladder: damage to the urothelial cells, causing hemorrhagic cystitis H2N M O-carboxyethylphosphoramide mustard 3 O P BIOTRANSFORMATION OF TERFENADINE (A PRODRUG ANTIHISTAMINE THAT IS PRONE TO CAUSE POLYMORPHIC VENTRICULAR TACHYCARDIA) INTO FEXOFENADINE (AN ACTIVE ANTIHISTAMINE) Terfenadine is INACTIVE as an antihistamine (it is a prodrug). As a K+ channel inhibitor, terfenanidine iinhibits repolarization of cardiomyocytes CH3 and is arrhythmogenic. It has caused C CH3 polymorphic ventricilar extrasystoles (called torsades de pointes) especially CH3 when the patient also received a CYP3A4 inhibitor drug, such as ketoconazole or erythromycin. Terfenadine [TELDANE] (withdrawn) OH HO C N (CH2)3 CH Hydroxylation CH3 OH HO C N (CH2)3 CH C CH2OH CH3 alcoholic metabolite Dehydrogenation Hydration Dehydrogenation OH HO C N (CH2)3 CH CYP CH3 C COOH CH3 carboxylic acid metabolite Ketoconazole Erythromycin CYP3A4 The carboxylic acid metabolite is ACTIVE as an antihistamine! Now it is marketed as fexofenadine [TELFAST] CYP3A4 inhibitors (e.g., erythromycin, ketoconazole) increased the arrhythmogenic effect of coadministered terfenadine and have caused several fatalities by precipitating polymorphic ventricular extrasystole (torsades de pointes). Now terfenadine is withdrawn and replaced with fexofenadine (TELFAST). AN EXCEPTIONAL WAY FOR ACTIVATION OF A DRUG: THE PRODRUG MINOXIDIL FORMS AN ACTIVE SULFATE CONJUGATE N N NH2 MINOXIDIL (prodrug, inactive as a vasodilator) N NH2 O PAPS Sulfotransferase N N NH2 MINOXIDIL-SULFATE N NH2 OH O S O O N + N NH3 N NH2 O O MINOXIDIL-SULFATE inner salt form (active vasodilator metabolite, excreted slowly) S O O The vast majority of sulfate conjugates are highly water-soluble (due to their anionic charge), pharmacologically inactive and readily excreted (e.g., acetaminophen-sulfate). In contrast, minoxidyl-sulfate, owing to inner salt formation which masks the + and – charges of this conjugate, is poorly water-soluble, pharmacologically active and slowly excreted. On a similar basis, the sulfate conjugate of 5-hydroxy-triamterene (the metabolite of the diuretic triamterene) is also an active and poorly water-soluble; in fact it may cause crystalluria by being precipitated out from the urine (see under Diuretics). AN EXCEPTIONAL WAY FOR ACTIVATION OF MORPHINE: BY CONJUGATION WITH GLUCURONC ACID AT THE 6-OH GROUP The superactive nature of Mo-6-glucuronide is explained by inner salt formation (like for minoxidyl-sulfate, see above) Morphine-3-glucuronide INACTIVE cannot form an inner salt Morphine ACTIVE Morphine-6-glucuronide SUPERACTIVE can form an inner salt COOH H O H OH H H OH HO H O HO 3 O OH UDP-GT O UDP-GA UDP-GT O NH 6 COOH NH O HO HO NH UDP-GA O H H OH H H OH OH H AN EXCEPTIONAL WAY FOR ACTIVATION OF EZETIMIBE: BY FORMATION OF A GLUCURONIDE THAT REACHES AND INHIBITS THE INTESTINAL CHOLESTEROL TRANSPORTER COOH O OH O OH OH OH UDP-GT F HO OH UDP-GA N O Ezetimibe F F N O Ezetimibe-glucuronide (EG) active metabolite produced in the enterocytes and the liver F Mrp-2 transporter the absorption of cholesterol decreases THE SERUM CHOLESTEROL LEVEL DECREASES EG inhibits the intestinal cholesterol transporter (NPC1L1) Bile, intestinal tract DETOXIFICATION OF ACETAMINOPHEN (PARACETAMOL) BY GLUCURONIDATION (OR SULFATION) AT ITS HYDROXYL GROUP AND TOXIFICATION OF ACETAMINOPHEN BY CYP2E1-CATALYZED DEHYDROGENATION TO A REACTIVE ELECTROPHILIC QUINONEIMINE (NAPBQI). O HN C Acetaminophen = Paracetamol O O CH3 toxication by dehydrogenation CYP2E1 N C (+) HN CH3 (+) protein OH O detoxication of paracetamol CH3 S NAPBQI OH C detoxication of NAPBQI glutathione (glu-cys-gly) UDP-GA UDP-GT O O HN C HN CH3 C CH3 S O glutathione OH glucuronic acid EXCRETION HEPATOCELLULAR NECROSIS You must know this! NAPBQI IS NORMALLY DETOXIFIED BY CONJUGATION WITH GLUTATHIONE. HOWEVER, WHEN ACETAMINOPHEN IS OVERDOSED, NAPBQI IS PRODUCED IN SO LARGE QUANTITIES THAT GLUTATHIONE BECOMES CONSUMED AND DEPLETED. THEN NAPBQI BINDS COVALENTLY TO THIOL GROUPS IN HEPATOCELLULAR PROTEINS, INACTIVATING THESE PROTEINS (enzymes, transporters, mitochondrial e-transport proteins, etc.) AND ULTIMATELY CAUSING LIVER NECROSIS. Alcoholists are hypersensitive to acetaminophen-induced liver injury, because (1) in the alcoholist’s liver the amount of CYP2E1 is increased, therefore more NAPBQI is formed, and because (2) in the alcoholist’s liver the amount of glutathione is decreased, therefore glutathione is more readily depleted, allowing NAPBQI to react with protein-thiols. NAPBQI = N-acetyl-para-benzoquinoneimine Part B. ALTERATIONS IN BIOTRANSFORMATION 1. BIOLOGICAL ALTERATIONS IN BIOTRANSFORMATION - summary Age-related alterations - Neonatal immaturity of biotransformation Genetic alterations: - Mutation of genes encoding drug metabolizing enzymes the mutant gene may code for an inactive or unstable enzyme protein POOR or SLOW metabolizer phenotype Such individuals may be hyperreactive to the drug if the active parent compound is slowly converted into inactive metabolite. - Multiplication of genes encoding drug metabolizing enzymes the gene in multiple copies codes for multiple amounts of enzyme protein EXTENSIVE or RAPID metabolizer phenotype Such individuals may be non-reactive to the drug if the active parent compound is rapidly converted into inactive metabolite. NEONATAL IMMATURITY OF BIOTRANSFORMATION summary UDP-GT immaturity Bilirubin Chloramphenicol Physiological jaundice (see FIG) Grey baby syndrome (see FIG) Immature glycine conjugation Benzyl alcohol Gasping syndrome (see FIG) Floppy infant syndrome (see FIG) Immature CYP, UDP-GT Diazepam UDP-GT immaturity: physiological jaundice, chloramphenicol-induced grey baby sy. Bilirubin (not excreted into bile) H N O CH H3C H N H3C CH 2 CH H N CH2 CH 2 CH2 CH2 CH2 C C HO O O Bilirubin diglucuronide (excreted into bile) H N CH CH 3 CH H N O O CH H N CH2 UDP-GA H3C H3C CH 2 CH CH 3 _ OOC OH O CH 2 CH2 CH2 CH2 C C O O OH H N CH2 UDP-GT O H N CH CH 3 CH O O COO HO OH Chloramphenicol glucuronide O2N CH CH CH2 OH O2N UDP-GA CH CH CH2 O C CHCl2 NH COO _ O OH UDP-GT _ OH HO OH CH2 CH 3 HO Chloramphenicol (CL) O NH O C CHCl2 HO OH OH O At high concentration, CL inhibits mitochondrial protein synthesis, causing: > cardiomyopathy > myopathy = GREY BABY SYNDROME Immature glycine conjugation: benzyl alcohol-induced gasping syndrome CH2 OH CYP ADH AOX BCS BC:GT Benzyl-alcohol BA accumulation acidosis gasping Cytochrome P450 Alcohol-dehydrogenase Aldehyde-oxydase Benzoyl-CoA-synthetase Benzoyl-CoA:glycine-N-acyl-transferase LOW CAPACITY STEP IN NEONATES ADH O O C C H C OH benzaldehyde S-CoA BCS BC:GT CoA-SH glycine AOX O2= O O benzoic acid (BA) benzoyl-CoA C NH CH2 COOH URINE benzoyl-glycine (hippuric acid) Note: The use of benzyl alcohol is prohibited in neonatology care units. Immature CYP, UDP-GT: diazepam-induced floppy infant syndrome (FIS) CH3 O N Cl diazepam active [VALIUM] N FIS developes in a baby whose mother received diazepam before delivery. T1/2= 40 hrs SLOW STEPS IN NEONATES CYP2C19 CYP3A4 H H O N H O N CYP Cl OH Cl N N O N UDP-GT UDP-GA O glucuronic acid Cl N T1/2= 8 hrs oxazepam active [SERAX] nordiazepam active oxazepam-glucronide inactive BLOOD ETHANOL CONC. (mg%) Immature alcohol dehydrogenase: delayed elimination of ethanol from the baby monkey whose mother received ethanol before delivery 140 NEONATAL MATERNAL 120 100 80 60 40 DELIVERY 20 0 0 50 100 150 200 250 300 350 TIME (min) Interpretation: Before delivery, ethanol elimination in the mother-fetus “unit” was entirely carried out by ADH in the maternal tissues. After delivery, the ethanol that remained in the baby can only be eliminated at a very slow rate because ADH is barely expressed in the neonatal tissues. This also happens to diazepam in floppy infant sy (see above). GENETIC ALTERATIONS IN BIOTRANSFORMATION I. Mutation of genes encoding drug metabolizing enzymes the mutant gene may code for an inactive or unstable enzyme protein POOR metabolizer (hyperreactive) phenotype 1. CYP deficiencies CYP2C9 deficiency Warfarin hydroxylation Adverse effects at regular dose: CYP2C19 deficiency Diazepam N-demethylation CYP2D6 deficiency Debrisoquin hydroxylation N-dealkylation of TCADs N-dealkyl. of antipsychotics Bleeding Prolonged sedation Pronounced hypotension Pronounced sedation, toxicity Tardive dyskinesia (involuntary movements, tics) 2. Carboxylesterase (pseudocholinesterase) deficiency Succinylcholine Prolonged paralysis 3. NAT2 deficiency slow acetylator phenotype Isoniazide Procainamide, hydralazine Neurotoxicity, hepatotoxicity Systemic lupus erythemathodes 4. Thiopurine methyltransferase deficiency 6-Mercaptopurine Myelotoxicity 5. Dihydropyrimidine dehydrogenase deficiency 5-Fluorouracyl Myelotoxicity II. Multiplication of genes encoding drug metabolizing enzymes the gene in multiple copies codes for multiple amounts of enzyme protein EXTENSIVE metabolizer (non-reactive) phenotype Multiplication of CYP2D6 gene ultrarapid metabolizer phenotype Psychotropic drugs, e.g., - TCADs (imipramine, nortriptyline), (most are CYP2D6 substrates) - SSRIs (fluoxetine, paroxetine) and - Others (clozapine, mianserine) are all ineffective at regular doses 2. CHEMICAL ALTERATIONS IN BIOTRANSFORMATION - summary Overexpression of enzymes by inducers Inhibition of enzymes by inhibitors Cytochrome P450 enzymes are often induced or inhibited by certain drugs or other chemicals. CYP inducers and inhibitors thus may significantly affect the fate and the effect of other drugs that are biotransformed by CYP enzymes. CYP superfamily organization: CYP families – labeled with numbers; only CYP1, CYP2 and CYP3 families are involved in biotransformation of drugs. (CYP7A, for example, is cholesterol 7hydroxylase, the rate limiting enzyme of bile acid synthesis.) CYP subfamilies – labeled with capital letters, e.g. CYP2B, CYP2C, CYP2E CYP family members – labeled with numbers after the letter of the subfamily, e.g., CYP2C9, CYP2C19, CYP2D6, CYP3A4 Of all the CYPs, CYP3A4 is the most abundant in human liver (35% of total) and in human small intestinal mucosa cells (80% of total intestinal CYP). Some drugs are biotransformed largely or exclusively by one CYP. For example: - Phenytoin and warfarin are hydroxylated by CYP2C9 - Halothane is oxidatively debrominated by CYP2E1 Other drugs may be biotransformed by several CYPs. For example: - Acetaminophen is dehydrogenated by CYP2E1 (mostly), CYP1A2 and CYP3A4 - Diazepam is N-demethylated by CYP2C19 and CYP3A4 - Dextromethorphan is O-demethylated by CYP2D6 and CYP3A4 A drug that is biotransformed by multiple CYPs, is typically less susceptible to pharmacokinetic interactions by CYP inhibitors, which often inhibit one specific CYP enzyme, and therefore CYPs that remain uninhibited may still eliminate the drug. ENZYME INDUCTION: Induction means increased synthesis of the enzyme protein. Induction is usually caused by increased gene transcription, which is mediated by ligand-activated transcription factors, such as aryl hydrocarbon receptor (AhR), constitutive androstane receptor (CAR) and pregnane X receptor (PXR). (see the table on the next page). The inducer is a drug or other chemical which binds as a ligand to the ligandactivated transcription factor (intracellular receptor) and activates it. The activated transcription factor (with its dimerizing partner) binds to a cognate sequence in the regulatory region of one or more CYP genes, thus promoting the transcription of such genes, ultimately increasing CYP protein synthesis. Inducers may induce multiple CYP members. For example, phenobarbital induces CYP2 members (except CYP2D) and CYP3A4, whereas ethanol induces only CYP2E1 (called ethanol-inducible CYP form; although CYP2E1 is not truly induced by ethanol but is stabilized by it.) THE MECHANISM OF CYP3A4 INDUCTION BY DRUGS: Increased CYPA4 gene transcription by activated pregnane X-receptor (PXR) Xenobiotic* (Ligand) Increased expression of CYP3A4 PXR Increased transcription of CYP3A4 mRNA Plasma membrane DNA ER6 Nucleus RXR *Ligands: rifampicin, phenobarbital, hyperforin (St. John's wort), etc. PXR = Pregnane X receptor RXR = Retinoid X receptor CYP3A4 (CYP3A7) CYP family CYP1 Drug substrates A1: barely expressed, unless induced. A2: caffeine, theophylline, tizanidine, ondansetron, acetaminophen Inducer chemicals Inducer Inhibitors (often receptors (TFs) competing substrates) Drugs: omeprazole AHR: A2: ciprofloxacine Others: PAHs (charcoalfluvoxamine broiled meat, smoking), Aryl cimetidine TCDD, indole-3-carbinol hydrocarbon acyclovir (in brussels sprouts, receptor broccoli, cabbage) CYP2 CYP3 (3A4) B6: cyclophosphamide, bupropion, ketamine C9: phenytoin, warfarin, tolbutamide C19: omeprazole, diazepam, fluvastatin D6: Psychotropic drugs: SSRI (citalopram, fluoxetine, paroxetine), TCAD (amitriptyline, clomipramine, desipramine, imipramine, nortriptyline), clozapine, mianserine, mirtazapine, venlafaxine. Cardiovascular drugs: -blockers (alprenolol, bufuralol, metoprolol, propranolol, timolol), encainide, flecainide, propafenone. Miscellaneous drugs: codeine, debrisoquine, dextromethorphan, phenformin E1: halothane, acetaminophen, dapsone, theophylline Benzodiazepines: alprazolam, clonazepam, clorazepate, diazepam, flurazepam, halazepam, midazolam, prazepam, triazolam. Calcium channel blockers: amlodipine, diltiazem, felodipine, isradipine, lercanidipine, nifedipine, nisoldipine, nitrendipine, verapamil. HIV antivirals: indinavir, nelfinavir, ritonavir, saquinavir. Immunesuppressive drugs: cyclosporine, tacrolimus, sirolimus. Macrolide antibiotics: clarithromycin, erythromycin. Steroids: estradiol, hydrocortisone, progesterone, testosterone. Statins: lovastatin, simvastatin, atorvastatin. Others: buspirone, codeine, dextromethorphan, methadone, taxol B6, C9, C19: phenobarbital rifampin B6: phenytoin E1: ethanol, isoniazide CAR: Constitutive androstane receptor (CYP2D6 is NOT inducible) PXR: rifampin, rifabutin phenobarbital Pregnane X phenytoin receptor carbamazepine nifedipine dexamethasone spironolactone lovastatin simvastatin hyperforin (an antidepressant in St John's wort) B6: clopidogrel ticlopidin C9: amiodarone (+D6), fluconazole C19: moclobemide D6: cimetidine quinidine terbinafine fluoxetine paroxetine E1: disulfiram A4: Drugs: Azole antifungals Macrolide antibiot. HIV protease inhib Gestodene (suicide inhibitor contrac. ster) Dietary chemicals: naringenin: a flvonoid, bergamottin: a furano -cumarin (both present in grapefruit juice) CONSEQUENCES OF CYP INDUCTION: 1. Enhanced inactivation of a drug after repeated administration Occurs when the drug is both a substrate and an inducer of a CYP; thus it accelerates its own biotransformation into inactive metabolites diminished effect (self-induced tolerance) e.g. Barbiturates (both substrates and inducers of CYP2 and CYP3A4) Meprobamate (both substrate and inducer of CYP) 2. Enhanced inactivation of some coadministered drugs after repeated administration of the inducer Occurs when the inducer drug induces a CYP which transforms the coadministered drug into inactive metabolite; thus the inducer accelerates the biotransformation of the coadministered drug into inactive metabolites diminished effect e.g. CYP2C9 inducers (phenobarbital, rifampin) + warfarin (anticoagulant, CYP2C9 substrate) INSUFFICIENT ANTICOAGULANT EFFECT of warfarin! THROMBOSIS, despite warfarin therapy! CYP3A4 inducers (phenobarbital, rifampin, phenytoin, carbamazepine) + an oral contraceptive (contains ethinyl estradiol, a CYP3A4 substrate) INSUFFICIENT CONTRACEPTIVE EFFECT of the contraceptive UNWANTED PREGNANCY, despite taking a contraceptive! CYP3A4 inducers (phenobarbital, rifampin, phenytoin, carbamazepine) + immunophyllin-binding immunosuppressive drug (e.g., cyclosporine A, tacrolimus, sirolimus; all are CYP3A4 substrates) INSUFFICIENT IMMUNOSUPPRESSION TRANSPLANT REJECTION, despite immunosuppressive therapy! 3. Enhanced toxification of a coadministered drug after repeated administration of the inducer Occurs when the inducer drug induces a CYP which transforms the coadministered drug into a toxic metabolite; thus the inducer accelerates the biotransformation of the coadministered drug into toxic metabolites increased toxicity e.g. CYP2E1 inducer (ethanol) + acetaminophen overdose (CYP2E1 substr.) increased formation of N-acetyl-p-benzoquinone imine (NAPBQI) HEPATIC NECROSIS! Another cause of increased susceptibility of drinkers to paracetamolinduced liver injury: lower hepatic GSH detoxication of NAPBQI CYP2E1 inducer (ethanol) + halothane anesthesia increased formation of trifluoroacetyl chloride the probability for halothane HEPATITIS may increase! CONSEQUENCES OF CYP INHIBITION: 1. Decreased inactivation of an active drug increased therap. effect: e.g. a CYP2C9 inhibitor (e.g., amiodarone) + the oral anticoagulant warfarin BLEEDING! a CYP3A4 inhibitor (e.g., itraconazole, clarithromycin) + the oral antidiabetic repaglinide (CYP3A4 substr.) HYPOGLYCEMIA! a CYP3A4 inhibitor (e.g., ketoconazole) + cyclosporine A (CsA; an immunosuppressive drug; CYP3A4 substr.) oral bioavailability and elimination of CsA Two consequences (Two birds are killed with one stone!): effectiveness (dose reduction saving on the drug) the antifungal ketokonazole prevents fungal infection of the immunosuppressed patient 2. Decreased inactivation of an active drug increased adverse effect: e.g. a CYP3A4 inhibitor (e.g., itraconazole, clarithromycin, bergamottin) + a CYP3A4 substrate statin (not pravastatin, fluvastatin, rosuvastatin) MYOTOXICITY! – see FIG. a CYP3A4 inhibitor (e.g., ketoconazole, erythromycin) + terfenadine (a withdrawn antihistamin; CYP3A4 substrate) POLYMORPHIC VENTRICULAR ES!– see FIG. a CYP1A2 inhibitor (e.g., ciprofloxacin, fluvoxamine) + tizanidine (a central muscle relaxant 2-receptor agonist; CYP1A2 substr.) EXCESSIVE SEDATION, HYPOTENSION! – see FIG. 3. Decreased activation of a prodrug decreased therapeutic effect e.g. a CYP2D6 inhibitior (e.g., fluoxetine or paroxetine) + tamoxifen (an antiestrogen prodrug, CYP2D6 substrate) decreased conversion into 4-hydroxytamoxifen (active antiestrogen) – see FIGURE Thus, CYP2D6 inhibitors can compromise the effectiveness of tamoxifen against estrogen-dependent breast cancers! 4. Decreased toxification of a drug decreased toxic effect e.g. CYP2E1 inhibitior (disulfiram) + halothan (an inhalation anesthetic, CYP2E1 substrate) decreased conversion into trifluoroacetyl chloride (reactive metab.) Thus, disulfiram pretreatment before halothane anesthesia could be used to decrease the likelihood of halothane hepatitis. GRAPEFRUIT JUICE (contains bergamottin which inhibits CYP3A4 as well as the OATP transporter-mediated hepatic uptake of statins) INCREASES THE PLASMA CONCENTRATION OF SIMVASTATIN (SV; A PRODRUG) AND ITS ACTIVE METABOLITE SIMVASTATIN ACID SEVERAL FOLD. (Lilja et al., Clin. Pharmacol. Ther. 64: 477-483, 1998.) 15 Simvastatin acid (ng/ml) Simvastatin (ng/ml) 30 25 grapefruit juice + SV 20 15 10 water + SV 5 0 10 grapefruit juice + SV 5 water + SV 0 0 2 4 6 8 10 12 14 16 18 20 22 24 0 2 4 6 8 10 12 14 16 18 20 22 24 Time (h) Time (h) BIOTRANSFRORMATION PATHWAYS FOR THE CHOLESTEROL-LOWERING DRUG SIMVASTATIN INTO ACTIVE SIMVASTATIN ACID BY PARAOXONASE AND INACTIVE METABOLITES BY CYP3A4 HO O HO COOH O O OH O Paraoxonase O O HOH Simvastatin (inactive) Simvastatin acid (active) CYP3A4 HO naringenin, bergamottin; itraconazole, verapamil O HO O O O HO O O O O O O O O HO OH 6'-hydroxy SV 3'-hydroxy SV H2C 6'-exomethylene SV CYP3A4 inhibitors, such as constiuents of grapefruit juice (naringenin and bergamottin) as well as drugs (itraconazole, verapamyl, erythromycin, etc.) may increase the myotoxicity of statins by diminishing their elimination by CYP3A4. OATP inhibitors, such as the grapefruit juice constituent bergamottin and the fibratetype triglyceride-lowering drugs (e.g., gemfibrozil), may also increase the myotoxicity of statins by diminishing their hepatic uptake mediated by OATP. BIOTRANSFORMATION OF TERFENADINE (A PRODRUG ANTIHISTAMINE THAT IS PRONE TO CAUSE POLYMORPHIC VENTRICULAR TACHYCARDIA) INTO FEXOFENADINE (AN ACTIVE ANTIHISTAMINE) Terfenadine is INACTIVE as an antihistamine (it is a prodrug) As a K+ channel inhibitor, terfenanidine iinhibits repolarization of cardiomyocytes CH3 and is arrhythmogenic. It has caused C CH3 polymorphic ventricilar extrasystoles (called torsades de pointes) especially CH3 when the patient also received a CYP3A4 inhibitor drug, such as ketoconazole or erythromycin. Terfenadine [TELDANE] (withdrawn) OH HO C N (CH2)3 CH Hydroxylation CH3 OH HO C N (CH2)3 CH C CH2OH CH3 alcoholic metabolite Dehydrogenation Hydration Dehydrogenation OH HO C N (CH2)3 CH CYP CH3 C COOH CH3 carboxylic acid metabolite Ketoconazole Erythromycin CYP3A4 The carboxylic acid metabolite is ACTIVE as an antihistamine! Now it is marketed as fexofenadine [TELFAST] CYP3A4 inhibitors (e.g., erythromycin, ketoconazole) increased the arrhythmogenic effect of coadministered terfenadine and have caused several fatalities by precipitating polymorphic ventricular extrasystole (torsades de pointes). Now terfenadine is withdrawn and replaced with fexofenadine (TELFAST). N N S S N Cl H N N Cl hydroxylation H N CYP1A2 NH Tizanidine N N NH S N N O Tizanidine, a centrally acting muscle relaxant, is rapidly biotransformed by CYP1A2. This is responsible for both its hepatic presystemic elimination (F = 0.7) and rapid elimination (T1/2 = 2.5 hrs) of tizanidine. Therefore, drugs that inhibit CYP1A2 (e.g., fluvoxamine, ciprofloxacin, rofecoxib) can increase the AUC of tizanidine several fold. At high level, tizanidine, which is a 2-receptor agonist like clonidine, can cause marked hypotension, bradycardia and sedation. N S N H N NH FLUVOXAMINE, CIPROFLOXACIN, ROFECOXIB OH Cl H N CYP1A2 HO hydroxylation CYP1A2 N Cl dehydrogenation NH N ------------------------------------------------------------------------------------------------------------------- O CH3 O N N CH3 CYP2D6 tamoxifen (prodrug) CH3 SSRI (e.g. fluoxetine paroxetine) CH3 -hydroxytamoxifen (active antiestrogen; used against breast cancer) OH CYP2D6 inhibitors (e.g., fluoxetine or paroxetine) can compromise the therapeutic effectiveness of tamoxifen against estrogen-dependent breast cancer because they inhibit the conversion of tamoxifen into 4hydroxytamoxifen, the active antiestrogen metabolite of tamoxifen. THERAPEUTIC USE OF ENZYME INHIBITORS CYP inhibitors: Ketoconazol – to increase effectiveness of cyclosporine (to save on the drug) Disulfiram – to decrease the likelihood of • halothane hepatitis or • acetaminophen-induced hepatic necrosis MAO inhibitors: MAO A inhibitors (e.g., moclobemide) – to treat major depression MAO B inhibitors (e.g., selegiline) – to treat Parkinson's disease COMT inhibitors: Entacapone – to treat Parkinson's disease XO inhibitors: allopurinol – to decrease uric acid formation (in gout) (BUT: it decreases the elimination and thus increases the toxicity of 6-mercaptopurine!) Alcohol dehydrogenase inhibitors: Fomepizol, ethanol – to treat methanol or ethylene glycol intoxication Aldehyde dehydrogenase (ALDH) inhibitors: Disulfiram – to induce „acetaldehyde syndrome” and thus promote alcohol withdrawal Note, disulfiram is not only an irreversible ALDH inhibitor, but a CYP inhibitor as well. Drugs with unwanted ”disulfiram-like effect”: - Some cephalosporins: cefamandol, cefoperazone (Their metabolite, 1-methyltetrazole-5-thiol, inhibits aldehyde dehydrogenase, like disulfiram does.) - Chloramphenicol - Metronidazole - Procarbazine