* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download High temperature superconductors are the materials with T c value
Shape-memory alloy wikipedia , lookup
Low-energy electron diffraction wikipedia , lookup
Superconducting radio frequency wikipedia , lookup
Heat transfer physics wikipedia , lookup
Superfluid helium-4 wikipedia , lookup
Multiferroics wikipedia , lookup
Strengthening mechanisms of materials wikipedia , lookup
Hall effect wikipedia , lookup
Geometrical frustration wikipedia , lookup
Giant magnetoresistance wikipedia , lookup
Crystal structure wikipedia , lookup
Scanning SQUID microscope wikipedia , lookup
Curie temperature wikipedia , lookup
Electromigration wikipedia , lookup
Glass transition wikipedia , lookup
Bose–Einstein condensate wikipedia , lookup
State of matter wikipedia , lookup
Semiconductor wikipedia , lookup
Condensed matter physics wikipedia , lookup
Ferromagnetism wikipedia , lookup
Superconducting magnet wikipedia , lookup
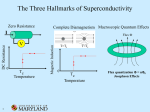
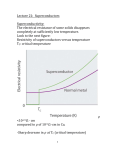
Superconductivity Superconductivity was discovered in 1911 by Heike Kamerlingh Onnes, who was studying the resistance of solid mercury at cryogenic temperatures using the recently-discovered liquid helium as a refrigerant. At the temperature of 4.2 K, he observed that the resistance abruptly disappeared. Superconductivity is an electrical resistance of exactly zero which occurs in certain materials below a characteristic temperature called critical temperature Tc. In subsequent decades, superconductivity was found in several other materials. In 1913, lead was found to superconduct at 7 K, and in 1941 niobium nitride was found to superconduct at 16 K. Alloys of Nb3Sn, Nb3Ge, Nb3Al etc show superconductivity & have Tc values of 18K, 20 K respectively. Superconductors have some unique properties • They have zero electrical resistance. • They carry current with no energy loss. • Meissner effect. • Characteristic critical temperature Tc Meissner effect Meissner and Ochsenfeld found that superconducting materials will not permit a magnetic field to penetrate their bulk. Superconducting materials expel all magnetic flux from their interior & so are forced out of a magnetic field. This is called Meissner effect & gives rise to levitation. Levitation occurs, when objects float on air. This can be achieved by the mutual repulsion between a permanent magnet & a superconductor. Superconductor exhibit perfect diamagnetism & it repels a magnet. Japanese have an experimental frictionless train ‘Maglev’ that floats above magnetic rails & has achieved speed of 500km/h . A magnet levitating above a high-temperature superconductor, cooled with liquid nitrogen. However until 1986, the highest temperature that a superconductor operated was 23K & this temperature was achieved by cooling using liquid Helium. This made the use of superconductor extremely expensive. A high temperature super conductor levitating above a magnet Critical temperature Tc- It is the temperature at which the resistance suddenly drops to zero . superconductivity occurs. It is also found that Tc changes in presence of a magnetic field. ie, As the applied field increases, the critical temperature drops. ie, a superconductor can be made non superconducting by the application of mag. field. The minimum value of field strength required to bring about superconductivity is known as critical field strength. Similarly, if the current in the superconductor exceeds a critical current, the superconductivity is destroyed. This is known as Silsbee effect An extremely high current can be passed through a very fine wire of superconductor. Thus small coils with large number of turns can be used to make extremely powerful high field electromagnet. Because of zero resistance the wire does not get hot. Because there is no current loss, once the current is flowing in the coil, it continues indefinitely. The major obstacle to the widespread use of low temperature superconductor has been the very low value of TC. The only way of attaining this value was to use liquid helium which is very expensive. Classification : Based on their critical temperature super conductors can be classified as Low temperature superconductor LTSC ( generally they need liquid Helium to be cooled under their critical temperature). Eg: Hg, Pb, Nb, High temp. superconductor HTSC ( having a Tc value > 77 K), - they reach the superconducting state by just cooling them with liquid nitrogen. Mechanism of low temperature superconductivity BCS Theory (due to Bardeen, Cooper & Schrieffer) The central concept of low temperature superconduction is the existence of ‘cooper pair’ i.e., a pair of electrons that exist on account of the two electron’s indirect interaction via the nuclei of the atoms in the lattice .This pairing is caused by an attractive force between electrons from the exchange of phonons & is slightly exceed the repulsion that they exert directly on one another. These pairs were supposed to move cooperatively through the lattice in such a way that the electron – phonon collisions were avoided. Because the cooper pair undergoes less scattering, it can carry charge freely through the solid & hence give rise to super conduction. Low temperature favors cooper pair formation & hence high temp superconductivity can not be predicted by BCS theory. A cooper pair may be considered as a particle with twice the mass and charge of an electron. In the super conducting state , the electric current is due to the motion of several cooper pairs in the same direction with the same velocity. Josephson Effect In 1962, Josephson predicted that if two superconducting metals were placed next to each other, separated only by a thin insulating layer, then a current would flow in the absence of any applied voltage. This effect is observed because if the barrier is not too thick, then electron pairs can cross the junction from one superconductor to the other without dissociating. High temperature Superconductors High temperature superconductors are the materials with Tc value well above 100+ 20K, greater than the boiling point of nitrogen (77K), but much lower than room temp. In 1986, a new high temperature superconductor with a Tc value of 35K was discovered. This compound is a mixed oxide in the Ba – La – Cu –O system formulated as La2-xBax Cu O4-y where x is between 0.15 &0.20 and y is very small. This compound has a perovskite structure, based on La2 Cu O4. Though La2 Cu O4 itself is nonconducting, superconductivity can be made by replacing 7.5 – 10 % of La3+ ions by Ba2+. [The perovskite structure is formed by compounds with formula ABO3. Examples include Ba TiO3, Ca Ti O3. The perovskite structure is cubic. A Ca2+ ion or Ba2+ is located at the body centered position, the smaller Ti4+ ions are located at each corner & O2- ions are located half way along each of the edges of the cube. Thus Ca2+ has a C.N of 12, since it is surrounded by 12 O atoms & Ti4+ are surrounded by 6 oxygen atoms ] History & progress of HTSC In 1986, a new high temperature superconductor. La2-xBax Cu O4-y discovered. . with a Tc value of 35K was LSCO (La2-xSrxCuO4) – i.e., when strontium was substituted for Ba, Tc value increases . Discovered in the same year • January 1987 , yttrium based Y Ba2 Cu3 O7-X (YBCO ) was discovered to have a Tc of 90 K. This is called 1-2-3 superconductor ( from the ratio of Y – Ba – Cu).This is prepared by Y2(CO3)3 + 4 Ba CO3 + 6 CuCO3 → 2 Y Ba2Cu3O7-X + 13 CO2 This compound has a Tc of 90 K which was significant for practical reasons. It allows liquid N2 to be used as coolant rather than liquid He. • 1988 - BSCCO (Ba – Sr – Ca – Cu – O) discovered with Tc up to 108 K, and TBCCO (T=thallium – Ba – Ca – Cu - O ) discovered to have Tc of 127 K. These are called warm super conductors. • The Bi–Sr–Ca–Cu–O system has three superconducting phases forming a homologous series as Bi2Sr2Can-1CunO4+2n+x (n = 1, 2 and 3). These three phases are Bi-2201, Bi-2212 and Bi-2223, having transition temperatures of 20, 85 and 110 K, respectively, where the numbering system represent number of atoms for Bi, Sr, Ca and Cu respectively.[31] • As of 2009, the highest-temperature superconductor (at ambient pressure) is mercury barium calcium copper oxide (HgBa2Ca2Cu3Ox), at 135 K and is held by a cuprate-perovskite material, rising up to 164 K under high pressure. • Recently, iron-based superconductors with critical temperatures as high as 56 K have been discovered. These are often also referred to as high-temperature superconductors. A small sample of high temperature superconductor BSCCO-2223 Examples Examples of high-Tc cuprate superconductors include La1.85Ba0.15CuO4, and YBCO (Yttrium-BariumCopper-Oxide), which is famous as the first material to achieve superconductivity above the boiling point of liquid nitrogen. Transition temperatures of well-known superconductors (Boiling point of liquid nitrogen for comparison) 4.2 Transition temperature (in kelvins) Material 133 HgBa2Ca2Cu3Ox 110 Bi2Sr2Ca2Cu3O10(BSCCO) 90 YBa2Cu3O7 (YBCO) 77 Boiling point of liquid nitrogen 55 SmFeAs(O,F) 41 CeFeAs(O,F) 26 LaFeAs(O,F) 20 Boiling point of liquid hydrogen 18 Nb3Sn 10 NbTi Hg (mercury) The crystal structure of HTSC Class Copper-oxide superconductors Iron-based superconductors Metallic low-temperature superconductors 1-2-3 super conductor has a perovskite structure .This comprises three cubic perovskite units stacked one on top of the other giving an elongated (tetragonal) unit cell. The upper & lower cubes have a Ba2+ ion at the body centered position and the smaller Cu2+ ions at each corner. The middle cube is similar but has Y3+ ion at the body centre. An ideal perovskite has the formula ABO3 & the stoichiometry of the compound is Y Ba2 Cu3 O9. O atoms are at the edge centre & hence in one unit cell , number of O atoms = 12 x 1/4 = 3. For three units 3x 3 = 9 . Cu atoms are at the corners .Thus in one unit cell, the number of Cu atoms = 8x 1/8 = 1. For the three units 3 x 1 = 3. Thus the formula is Y Ba2 Cu3 O9. But in 1 – 2 – 3 super conductor, the actual formula found is Y Ba2 Cu3 O7- X . There is a massive oxygen deficiency and about one quarter of oxygen sites in the crystal are vacant. These occur between adjacent Cu atoms in the chain along the ‘c’axis. The vacancies are in the yttrium atom plane. There are also vacancies between copper atoms along ‘a’ axis in the Cu – O planes of Ba atoms. (Deficiency=8x1/4=2. Ie, 2 O atoms lost ). ) Cu Ba O7-X Cu Y O7-X Cu Ba O7-X The following generalizations can be done. 1. All these super conductors are related to Perovskite structure 2. Superconductivity occurs in the Cu O2layer. 3. Many, but not all warm superconductors contain Cu. Two features of copper are that it can exist in three oxidation states (+1), (+2) & (+3) and Cu II forms many tetragonally distorted octahedral complexes. Both these factors are important. In the La2Cu O4 compounds some Ba2+ ions substituted for La3+. To balance the charge, some Cu II atoms changes into Cu III. Another point to note is that if we assign the usual oxidation number (Y3+, Ba2+ , O2- ) ,then the average oxidation number of Cu turns out to be 2.33. Thus it is inferred that Y Ba2 Cu3 O7 is a mixed oxidation state material that contains Cu II & Cu III. Super conductivity in this system is thought to involve the transfer of electrons from Cu II to Cu III. But if the process involves two electrons, it could involve electron transfer from Cu I to Cu III 4. Oxygen deficiency seems to be critical. In ideal perovskite, Cu is octahedrally surrounded by 6 O atoms . But when an oxygen deficiency occurs, then the two Cu atoms may interact directly with each other. Interactions such as Cu II - Cu III occur by transferring an electron between the two Cu atoms. 5. In the 1 – 2- 3 super conductor, if all three sections were based exactly on perovskite unit cells, we expect O atoms in the middle of each cube edge. This give a formula of Y Ba2 Cu3 O9. This formula is improbable because it gives an average oxdn state for the three copper atoms of 11/3 implying that the unit cell contains both Cu III & Cu IV, which is unlikely as Cu IV complexes are extremely rare . The unit cell in fact contains only seven O atoms Y Ba2 Cu3 O7 . The O atoms on the vertical edges of central cube are not there & there are also two, missing from both top and bottom faces. A unit cell containing 7 O atoms has an average oxidation state of 2.33 indicating the presence of Cu II & Cu III in the unit cell. 6. La2Cu O4 has no superconductivity, because it is antiferromagnetic. This crystal contains Cu2+ ions & the unpaired electrons on Cu ions align anti parallel through out the structure. This interaction locks the electron to the lattice stopping both conductivity & superconductivity. When some Ba2+ ions are introduced in the lattice replacing La3+, some charge compensation has to be made. Thus for every Ba 2+ ion ,one Cu2+ must be oxidized to Cu3+ & this break up the alignment of unpaired spins . When the average oxidation state of Cu reaches a critical value of 2.2, the anti ferromagnetism disappears & super conductivity appears. If the super conductor 1 – 2- 3 is made more deficient in oxygen(Y – Ba2 – Cu3 O6.5), the super conductivity drops to 60 K & at Y – Ba2 – Cu3 O6 the superconductivity disappears. Applications The uses of superconductors are based on four main properties 1. Meissner effect 2. Lack of electrical resistance 3. The ability to create superconducting magnets 4. The Josephson effect Due to Meissner effect, Levitation is readily induced in super conducting material, which have a variety of applications including transport systems with ‘Maglev’ trains Superconducting magnets are some of the most powerful electromagnets known. They are used in MRI and NMR machines, mass spectrometers, and the beam-steering magnets used in particle accelerators. They can also be used for magnetic separation, where weakly magnetic particles are extracted from a background of less or non-magnetic particles, as in the pigment industries. In the 1950s and 1960s, superconductors were used to build experimental digital computers using cryotron switches. More recently, superconductors have been used to make digital circuits based on rapid single flux quantum technology and RF and microwave filters for mobile phone base stations. Superconductors are used to build Josephson junctions which are the building blocks of SQUIDs (superconducting quantum interference devices), the most sensitive magnetometers known. SQUIDs are used in scanning SQUID microscopes and Magnetoencephalography. Series of Josephson devices are used to realize the SI volt. Depending on the particular mode of operation, a Josephson junction can be used as a photon detector or as a mixer. Promising future applications include high-performance smart grid, electric power transmission, transformers, power storage devices, electric motors (e.g. for vehicle propulsion, as in vactrains or maglev trains), magnetic levitation devices, fault current limiters, nanoscopic materials such as buckyballs, nanotubes, composite materials and superconducting magnetic refrigeration