* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download The distribution of retino‐collicular axon terminals in rhesus monkey
Neuroplasticity wikipedia , lookup
Process tracing wikipedia , lookup
Convolutional neural network wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Neuroesthetics wikipedia , lookup
Pattern recognition wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Cortical cooling wikipedia , lookup
C1 and P1 (neuroscience) wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
The Distribution of Retino-Collicular Axon Terminals in
Rhesus Monkey
J. G. POLLACK
AND
T. L. HICKEY ' '
'School of Optometry/The Medical Center and Neuroscience Program, Uniuersity of
Alabama in Birmingham, Birmingham, Alabama 35294
ABSTRACT
The retino-collicular projections in rhesus monkeys were studied using standard autoradiographic and degeneration techniques. A computer
based technique was developed which provided a flattened visual display of the
retinal projection onto the entire superior colliculus, quantified the area covered by such projections for different segments of the colliculus and showed
how this morphological pattern varied with depth beneath the collicular surface.
In the anterolateral third of the colliculus (i.e., the foveal representation) the
retinal projection was light, confined t o a narrow region of the superficial gray
and contributed primarily by the contralateral eye. In the remaining binocular
segment of the superior colliculus the retinal projections showed a marked
degree of local patterning, that in many instances appeared as bands of label.
By combining eye removal and eye injection procedures in a single animal and
comparing adjacent sections processed for autoradiography and stained for degeneration, it was possible to assess the amount of overlap between retino-collicular projections from the two eyes. These experiments showed that total segregation of retinal afferents does occur in the monkey superior colliculus, but
what occurs more often is a situation where the density of inputs from the two
eyes varies reciprocally as one moves across the part of the colliculus that represents the binocular visual field.
Recent autoradiographic studies (Graybiel,
'75, '76; Hubel et al., '75) have demonstrated
that the retino-recipient layers of the cat and
monkey superior colliculus are organized partly on the basis of ocularity. In the cat superior
colliculus ipsilateral retinal projections are
discontinuous, forming a series of patches or
"puffs" of label when viewed in single frontal
sections. However, when serial sections are
viewed in sequence, it is evident that the individual patches of label seen on single sections
are, in fact, a part of longitudinal bands that
traverse the surface of the colliculus. Graybiel
("76) has suggested that these bands form alternating left eye-right eye stripes, somewhat
analogous to the ocular dominance columns
seen in the visual cortex (Hubel and Wiesel,
'68, '69, '72; Shatz et al., '77; LeVay et al., '78).
In the monkey, the existence of a similar patterning of retino-collicular input has been
suggested (Hubel et al., '751, but has not been
demonstrated conclusively. Furthermore,
even if retino-collicular projections from the
J. COMP. NEUR.(1979) 185: 587-602.
two eyes do form bands in the monkey, the
amount of overlap between the ipsilateral and
contralateral projections is still unclear.
In an attempt to answer these questions, we
undertook an extensive analysis of the pattern of retino-collicular projections in seven
rhesus monkeys. We utilized autoradiographic
techniques, supplemented by computer reconstruction, to examine the distribution of retinal axon terminals in the superior colliculus
as well as combined autoradiographic and
degeneration techniques to determine the
degree of overlap between the inputs from the
two eyes. In general, our findings show that
each colliculus receives a significant projection from both eyes. In all cases the contralatera1 eye is more strongly represented, a t least
in terms of the volume of collicular tissue innervated. In the anterolateral one-third of the
Present address: Lt. J. Pollack, MSC. USNR, NAMI, NAS, Pensacola, Florida 32508.
a Send reprint requests to: Lk.T. L. Hickey,School of Optometry,
University of Alabama in Birmingham, Birmingham, Alabama
35294.
587
588
J. G. POLLACK AND T. L. HICKEY
TABLE 1
Summary of animals used
Monkey
Right eye
Survival
1
2
3
4
Removed
5
6
Removed
ladays
5 days
I
Left eye
Survival
H3Proline(l.OmC)
H3Proline(1.2 mC)
H 3Proline (500 pC)
H3Leucine (250gC)
H3Proline(250 pC)
H3Leucine (250uC)
H3Leucine (500hC)
H3Proline(500pC)
H3Leucine (500 pC)
20 hours
36 hours
Coronal
Coronal
20 hours
Parasagittal
20 hours
Coronal
20 hours
20 hours
20 hours
Coronal
Oblique
Tangential
superior colliculus, the area representing approximately the central 10" of visual field
(Cynader and Berman, '721, the retinal projection, as demonstrated by the autoradiographic
technique, is light in some monkeys and totally absent in others. In the part of the colliculus representing more peripheral regions
of the binocular visual field, the input from
the two eyes is arranged in bands somewhat
analogous to those seen in the cat superior colliculus (Graybiel, '75, '76). More peripheral regions of the visual field (i.e., the monocular
crescent) form a fairly continuous projection
onto the posteromedial surface of the contralateral superior colliculus.
MATERIALS AND METHODS
A summary of the experimental protocol followed for each of the seven adult rhesus monkeys used in the present study is shown in
table 1. All animals received an intraocular
injection of a tritiated amino acid (doses ranging between 500 and 1,200pCi). For five of the
seven animals this was the only experimental
intervention. The brains from these animals
were embedded in paraffin, sectioned (14 p ) in
either the coronal, parasagittal or tangential
plane, mounted on glass slides, dipped in photographic emulsion (Kodak NTB-2) and exposed for one month a t 4°C. These sections
were then developed and stained with cresyl
violet. Camera lucida drawings, made from
the coronal and parasagittal sections, were
used in making t h e computer assisted reconstructions described below.
Two animals had one eye removed 5 to 12
days before the other eye was injected. The
brains from these animals were cut frozen (25
p ) with every fourth and fifth coronal section
processed for autoradiography and stained for
degeneration (Wiitanan, '69), respectively.
Since two adjacent sections then showed the
Plane of section
distribution of axon terminals from both eyes,
it was possible to study the overlap of retinal
input from the two eyes by aligning the sections on common landmarks.
--
Computer assisted reconstructions
In an attempt to reconstruct the morphological pattern of retino-collicular input, we developed a computer based technique which:
provides a flattened visual display of the retinal projection onto the entire superior colliculus, quantifies the area covered by such
projections for different segments of the col-
A.
8.
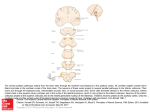
Fig. 1 Line drawing illustrating the method used to
digitize and transform the anatomical data for computer
plotting. A. The outlines of the collicular surface and
radioactive labeled region are first drawn with the help of
a camera lucida. This drawing is then digitized using a
Graph-Pen digitizer. The important points and distances
used in the transformation are shown. B. The collicular
surface and retinal projection shown in A a s it appears
after the transformation. For additional information see
text.
RETINO-COLLICULAR PROJECTIONS IN RHESUS MONKEYS
liculus and shows how this morphological pattern varies with depth beneath the collicular
surface.
Initially, every third section was drawn (63
x under darkfield illumination with the help
of a camera lucida. Points of uncertainty were
always checked using brightfield illumination
and a higher magnification to insure that the
silver grains were distributed around cell
bodies. Each drawing consisted of the outline
of the collicular surface, a common landmark
(e.g., the midline) and enclosed areas corresponding to the regions covered by dense terminal label. These drawings were traced using
a Graph-Pen digitizer and the data stored on
magnetic tape for further processing. The
data is stored in the form of X and Y coordinates where X is the distance from the origin
(0)along the collicular surface and Y is depth
below the collicular surface.
In order to flatten the colliculus, the distance between successive digitized points on
the collicular surface, such as Q in figure l A ,
is calculated from the origin (0).The distance
OQ (Xin fig. 1A) is then plotted as a straight
line. This line represents the flattened surface
of a segment of the colliculus with absolute
distance preserved. We then reconstructed the
perimeter points (P) of the labeled areas
beneath the surface. This was accomplished
by finding for each point P the point Q on the
surface which was closest. The distance between P and Q corresponds to the Y coordinate
for the flattened representation.
-
RESULTS
The computer based technique makes available two types of visual display. First, a flattened reconstruction of the entire colliculus
can be obtained (for example, see fig. 2). This
type of plot shows a flattened reconstruction
of the retinal projection to each of the sections
studied. To help in viewing the overall pattern
of retino-collicular projection the individual
sections have been lined up to show the colliculus as viewed from above. Starting a t the
top of t h e reconstruction shown in figure 2,
one can seen how the pattern of the retino-collicular projection, as determined by the distribution of silver grains, changes as one
moves from the rostra1 to the caudal pole of
t h e colliculus. Although depth information is
also contained in this reconstruction; i.e., in
terms of the thickness of the blackened areas,
this information is difficult to interpret. To
assist in this interpretation, a second type of
589
reconstruction was developed. This reconstruction shows the labeled regions as before,
except that only one depth beneath the collicular surface is illustrated on any given plot
(for example, see fig. 4). Since these reconstructions represent only one depth, they are
drawn using lines rather than solid, blackened
areas. Each line represents a scan a t a given
depth across one section with consecutive
lines representing a series of sections through
the rostral-caudal extent of the colliculus.
Such plots can be obtained for any given depth
below the collicular surface.
Relative volume occupied by ipsilateral and
contralateral retinal projections
In the rhesus monkey, each visual hemifield
is projected topographically onto the surface
of the superior colliculus (Cynader and Berman, '72). The central visual field is represented anterolaterally and the lateral (monocular) field posteromedially. We assessed the
distribution of label, in terms of relative labeled volume of collicular tissue, by dividing
the colliculus into zones approximating the
central 10" of the binocular visual field (CF)
(Wilson and Toyne, '70; Cynader and Berman,
'72; Hubel et al., '751, the remaining binocular
visual field (BF) representation (as defined by
the caudal boundaries of the ipsilateral retinal projection) and the monocular visual field
(MF) representation. Since the borders of
these zones have been defined quite arbitrarily, the relative volume of labeled tissue in any
one zone is only approximate.
Figure 3A shows the labeled volumes averaged over three animals. When the total
volume of one superior colliculus receiving
input from either eye is normalized to loo%,
4%of the labeled tissue is located in the CF,
82%in the BF and 14% in the MF. The relative
inputs of the two eyes are shown in figure 3B.
In the CF only one-fourth (1%)of the labeled
tissue receives input from the ipsilateral eye.
In the BF, where 82% of all labeled tissue is
found, the relative inputs are again biased in
favor of the contralateral eye by a factor of 2
(56%)to 1 (26%).The MF of the superior colliculus contains 14% of the total labeled
volume, all of which is due to projections from
the contralateral eye. The colliculus is therefore dominated by contralateral input in
terms of the volume of collicular tissue innervated.
It is important to note that for this analysis,
and for the plots shown in figures 2 and 4, we
590
J. G. POLLACK AND T. L. HICKEY
Fig. 2 Plot showing a series of transformed camera lucida drawings made from coronal sections taken
from throughout the rostral-caudal extent of the right superior colliculus in a monkey that received a left
eye injection. In this plot all three dimensions are represented; the thickness of the blackened areas corresponding to the depth dimension. A well-definedlandmark, the optic disc representation (OD), can be seen as
a large, oval break in the contralateral projection. The figure is aligned on the midline. M is medial, A is anterior. The overall shape of the colliculus is distorted since including the depth information required elongating the plot along the anterior-posterioraxis.
RETINO-COLLICULAR PROJECTIONS IN RHESUS MONKEYS
Anterior
A.
Lateral
Medial
Posterior
8.
Contralateral
Ipsilat era1
Posterior
Fig. 3 The relative volume of collicular tissue receiving retinal projections. In each case the colliculi were
first divided into segments approximating the central 10" of visual field (CF), the remaining binocular visual
field (BF), and the monocular field (MF). Then the volume of tissue receiving a retinal projection was determined for each colliculus following an injection of radioactively labeled amino acid into one eye. The volumes
of labeled tissue in the colliculi ipsilateral and contralateral to the injected eye were then summed, the resulting value being interpreted as the total volume of tissue occupied by the retinal projections onto one colliculus. In A, this volume was then set a t 100%and the volume contained in each of the three segments calculated relative to this 100%.In B, the relative input from each eye is shown separately. Although the ipsilateral and contralateral projections are shown in two collicular outlines the values given still correspond to
the relative volumes within one colliculus.
59 1
592
J. G. POLLACK AND T.
have been concerned with the dense patches of
label that are quite obvious using darkfield
illumination. In many instances light label
could be seen extending between patches of
dense label, especially contralateral t o the
injected eye. While this lighter label is only
slightly above background level, it may correspond t o a sparse retinal projection. If such
areas had been included in the volume calculations, the relative amount of tissue innervated by the contralateral eye would have
been even greater.
Distribution of retinal projections
The projections of the ipsilateral and the
contralateral retinal fibers differ, both in
their distribution across and in their depth
below, the collicular surface (Graybiel, '75,
'76; Hubel et al., '75). The projection of the
central retina onto the anterolateral part of
the superior colliculus has been the subject of
some discussion in the literature. In this region, some studies using both degeneration
and autoradiographic techniques have failed
to find evidence of retinal input (e.g., Brouwer
and Zeeman, '26; Bunt et al., '75; Wilson and
Toyne, '701, while other studies have found a
light, though definite projection (Hendrickson et al., '70; Hubel et al., '75).
We have identified label of retinal origin in
the anterolateral superior colliculus for some,
but not all, of our monkeys. In those animals
where a projection could be found (as in the
case of fig. 4) the label was light, diminished
rapidly as the anterolateral border of the colliculus was encountered, and was confined to
the most superficial parts of the superior colliculus. These animals were our most heavily
labeled monkeys. Consequently, the degree of
labeling in the foveal representation may be,
to some extent, a function of the amount of
radioactive tracer injected. Where label was
found, using the autoradiographic technique,
the majority of label was contributed by the
contralateral eye, that from the ipsilateral
eye being less distinct or not present a t all. In
all cases the part of the lateral geniculate nucleus receiving input from central retina
(Malpeli and Baker, '75) was well labeled,
demonstrating that differences in the presence of label in the anterolateral superior colliculus are not due to uneven retinal uptake of
radioactive material. In the two animals
which also had one eye removed, we were not
able to see any clear signs of degenerating
axon terminals in this area.
L. HICKEY
The majority of the retinal projection from
both eyes, in terms of the volume of collicular
tissue labeled, represents the region of visual
field from approximately 10" off the vertical
meridian t o the outer boundaries of the binocular visual field (BF). Within this region,
both eyes are represented by a patchy distribution of label on individual sections. When
these patches are traced over serial sections, a
banding pattern becomes evident that is similar to, but not as distinct as, that reported by
Graybiel ('75, '76) for the cat superior colliculus. In addition, a t least a partial laminar
segregation of label becomes apparent as the
ipsilateral retinal projection is most commonly displaced below that from the contralateral
eye.
Figure 4 represents a series of depth profiles
for the ipsilateral and contralateral retinal
inputs. The superior colliculus is viewed from
above, and each consecutive computer plot
shows the next level in depth - as if layers of
an onion were being removed. The lines indicate the presence of dense label. Clearly, some
label from each eye appears at each level examined, however, a partial laminar segregation does appear. The segregation is most clear
in the region between the optic disc representation and the anterolateral edge of the
colliculus. Here, ipsilateral label is found to be
heavier from 70 p to about 150 p below the
surface, whereas the contralateral input is absent below 110 p. Hence, the two projections
are somewhat unbalanced a t the most superficial level due to the predominance of the contralateral input, then combined at an intermediate level, and finally segregated at the
deeper level due to the absence of a contralatera1 input.
The monkey illustrated in figure 4 shows
one region of label that, like the foveal projection, occurred in some of our more heavily labeled monkeys. While there is a tendency for
the ipsilateral projection to be located superficially in lateral parts of the superior colliculus, we have also seen a very superficial
tier of label in medial parts of the colliculus.
Such superficial projections are always accompanied by a deeper ipsilateral projection.
Examples of such tiering of ipsilateral input
can best be seen in figure 7B (arrow), but is
also apparent in figure 4. A very similar projection in the cat superior colliculus has previously been described by Graybiel('76) and was
mentioned for the monkey superior colliculus
by Hubel e t al. ('75).
RETINO-COLLICULAR PROJECTIONS IN RHESUS MONKEYS
Near the medial and lateral borders of the
superior colliculus differences in the distribution of ipsilateral and contralateral projections were even more pronounced. On the contralateral side, the medial and posterior borders of the superior colliculus were covered by
an almost solid sheet of label forming a crescent some 1,200 to 1,300 p wide. This crescent,
which is largely monocular, is more discontinuous superficially, giving a scalloped appearance in coronal sections. On the ipsilateral side, the collicular borders were, for the
most part, devoid of label. However, three distinct patches of label consistently appeared
here. Two of these have been previously described by Hubel et al. (‘75). At the lateral
border of the ipsilateral superior colliculus
and just anterior to the level of the optic disc
representation is found an isolated patch of label 50 p wide and some 1,000 to 1,200 p long
with a depth distribution from 50 p to almost
200 p (see arrow 1 in fig. 4). This relatively
isolated projection has been found in all our
animals. On the medial border, an anteriorly
placed patch of label is present, which becomes continuous with other label posteriorly
(see arrow 2 in fig. 4). This patch is also about
1,000 p in length and is present throughout
the depth of the ipsilateral superficial gray.
Finally, a third, not previously described,
patch of label appears on the medial border a t
the level of the optic disc representation (see
arrow 3 in fig. 4).This patch is connected to
the main projection a t its anterior end and
does not seem to be as prevalent throughout
the depth of the superficial gray.
In the posteromedial segment of the superior colliculus the ipsilateral retinal projection
is almost totally absent below 70 p. This region receives a strong projection from the contralateral eye and most certainly corresponds
to the monocular segment of the superior colliculus (Cynader and Berman, ’72).
Finally, there is one other area where the ipsilateral and contralateral projections appear
to be totally segregated. This area, which can
reasonably be interpreted as the optic disc representation, can be seen quite clearly in
figures 2 (OD) and 4 (arrow and outlined
area). In figure 2, the optic disc representation
appears as a break or “hole” in the contralateral projection in the caudal half of the colliculus. Although other smaller “holes” in the
contralateral projection can be seen, they are
never as consistently placed nor as large as
the break we have interpreted to be the optic
593
disc representation. Almost identical representations of the optic disc have been seen in
every brain studied. In addition, when the ipsilateral retinal projection is studied, a corresponding solid projection (arrow in fig. 4) can
be seen in this region. While this projection is
greatest superficially, there is some ipsilatera1 input to this region throughout the
depths shown in figure 4.
Pattern of retinocotlicular projections
As suggested by Hubel et al. (‘79, the retinocollicular projection in rhesus monkeys is
organized in a manner similar to that seen in
the cat (Graybiel, ’75, ’76). That is, the retinotectal projection exhibits a marked degree of
local patterning that is similar to the banding
seen in the cat superior colliculus; although
such bands in monkeys are considerably more
variable and much less distinct. While the
overall orientation of the band-like patches of
label in the monkey superior colliculus is longitudinal, the pattern is complex. Figure 5
shows an example of the complexity of the
banding pattern for the ipsilateral retinal projection to a region just anterior and medial t o
the optic disc representation. This photomicrograph is a mosaic composed of several darkfield photomicrographs of serial sections cut
tangential to the surface of the colliculus.
Thus, the resulting photomicrograph represents a view of the top of the superior colliculus. The comparable region in the colliculus contralateral to t h e injected eye
showed a similar, though much less striking,
pattern.
Overlap of retinal projections
Given the rather complex, but definite, pattern of retino-collicular projection seen in
figures 4 and 5, the question immediately
arises as t o what extent the inputs from the
two eyes are segregated by having the contralateral label confined to the spaces on the ipsilateral side and vice versa. We have attempted
to answer this question in two ways. First, in
figure 6, we have redrawn one of the line plots
(90p) shown in figure 4.However, now the ipsilateral and contralateral projections have
been superimposed using the optic disc representation for alignment. In addition, the isolated contralateral projections are shown in
green and the isolated ipsilateral projections
in red. Region of overlap are shown in black.
This figure shows that the retino-collicular
projections from both eyes overlap in some
DEPTH 50 U
Fig. 4 The distribution of the retino-collicular projections, as seen on a series of coronal sections, is shown for several depths beneath the collicular surface. The ipsilateral projection is shown in the left column, the contralateral projection in the right column. The depth beneath the collicular surface is shown at the upper right hand corner of each depth
pair. In all cases the outlines of the colliculus are approximate and are included only to assist in making comparisons between ipsilateral and contralateral projections. For each pair medial is to the center, anterior a t the top. The optic disc
595
RETINO-COLLICULAR PROJECTIONS IN RHESUS MONKEYS
n
DEPTH 170 U
representations in the contralateral (outlined area) and ipsilateral (arrow) superior colliculi are best seen 90 f i below
the collicular surface. A few examples of isolated ipsilateral input can be seen in areas that would normally be considered the monocular segment (for example see the line plot for 110 p ) . It is possible that these projections are aberrant. However, it is also possible that these few patches of silver grains do not correspond to retinal projections at all,
but simply represent regions of high background label.
596
J. G. POLLACK AND T. L. HICKEY
Fig. 5 Darkfield photomontage of tangentially cut sections (see drawing upper right) through the superior colliculus ipsilateral to the injected eye. The montage shows a region approximately 1 mm diameter
located anterior and medial to the optic disc representation.
areas and remain separate in other areas.
Viewing the projections in this way also shows
that both the overlapping and segregated projections are organized in a band-like pattern.
While this pattern is complex, all of the bands
appear to intersect the monocular segment a t
approximately right angles.
This technique for comparing input to each
colliculus is of limited use, however. Perfectly
coronal sections, i.e., sections that cut through
exactly the same regions of both hemispheres,
are extremely difficult to obtain. Furthermore, the optic disc representation forms the
only reliable landmark on which sections from
the two colliculi can be aligned. Since the
optic disc representation is seen clearly in
only a few of the depth plots shown in figure 4,
it is impossible to make accurate comparisons
for all levels of retinal input. However, if comparisons between ipsilateral and contralateral
projections can be made in a single colliculus,
then the plane of section becomes relatively
unimportant. To do this, we have made comparisons in animals who had one eye removed
several days prior to having their remaining
eye injected with a radioactively labeled
amino acid.
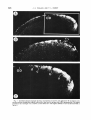
Figure 7A shows a darkfield photomicrograph of a coronal section through the contralateral colliculus a t the level of the optic disc,
and figure 7B the corresponding section on the
ipsilateral side. Inspection of the two photomicrographs suggests t h a t the retinal inputs
are segregated in some regions. However, to
RETINO-COLLICULAR PROJECTIONS IN RHESUS MONKEYS
597
598
J. G. POLLACK AND T. L. HICKEY
Fig. 7 Darkfield photomicrographs showing contralateral (A and C) and ipsilateral (B) projections onto
coronal sections through the superior colliculus at the level of the optic disc (OD)representation. The region
enclosed in the rectangle in A is shown in more detail in C. The regions labeled a-d are further described in
figure 8.
RETINO-COLLICULAR PROJECTIONS IN RHESUS MONKEYS
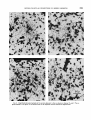
Fig. 8 Brightfield photomicrographs of the section adjacent to the one shown in figures 7A and C. The regions labeled a-d in figure 7C are shown here on the adjacent section stained for degeneration.
599
600
J. G. POLLACK AND T. L. HICKEY
verify this, it is necessary to examine the next
section in the series stained for degeneration
(fig. 8a-d). Figure 7C is a more highly magnified view of the outlined area in figure 7A.
Each of the regions labeled a-d are also shown
on adjacent sections stained to show degenerating axon terminals (fig. 8). In three of
these regions (a, b, and d) the contralateral
retinal projection, as measured by the distribution of silver grains, was markedly
reduced. In every instance, examination of the
adjacent section showed that degenerating
axon terminals filled each of the regions under
study. It is important t o note, however, that
while the degenerating axon terminals were
most prevalent near the center of each of
these regions, other examples of terminal degeneration could be seen extending into the
adjacent areas, and thus, overlapping with a
dense contralateral projection. In the remaining region (c) marked in figure 7C the contralateral retinal projection is only slightly
reduced. However, figure 8c shows that the ipsilateral retinal projection also extends into
this region, again overlapping with the contralateral input. These findings show that
many areas of the superior colliculus receive
input from both eyes, a situation that is also
apparent in the line plot shown in figure 6.
There are other regions in the superior colliculus where the dense label corresponding to
the ipsilateral and contralateral retinal projections do not overlap. The two most obvious
examples of this are the optic disc representation, where the ipsilateral projection fills a
void in the contralateral projection, and the
monocular segment, where the contralateral
projection exists in isolation. Figure 6 shows
that, a t least for the projection to a region of
the colliculus 90 p below the surface, the ipsilateral and contralateral inputs are segregated in many other areas. It must be emphasized, however, that these line plots show
only the areas that contained the dense
patches of terminal label. As mentioned
before, there were other areas, especially contralateral to the injected eye, where more
sparsely distributed label could be seen between the dense patches of label. If these regions of light label do, in fact, represent retinal projections, then there would be far more
overlap of retinal projections than shown in
figure 6. The findings in the animals having
one eye removed and the other eye injected
add further support to this hypothesis. In general, in areas where the silver grains were
severely reduced or absent, the density of degenerating axon terminals tended to be
greatest. As the number of silver grains increased in adjacent areas the number of degenerating axon terminals appeared to decrease.
In a few instances, we were able to find
small areas containing cell bodies that appeared not to receive retinal input from either
eye. Such areas were not confined to any one
part of the superior colliculus, sometimes even
occurring in the monocular segment. It is possible that such cell areas receive input from
other parts of the brain. However, it is also
possible that cells in these areas have dendritic appendages that extend into nearby regions that do receive input. For the most part,
our detailed analysis of the extent of overlap
between retinal projections has been confined
to the areas near the level of the optic disc representation. We were unable to examine the
complimentarity of inputs to the anterolateral superior colliculus (foveal representation) since our staining techniques consistently failed to demonstrate any degenerating
axon terminals in this region.
DISCUSSION
The findings reported here confirm and extend many of the results previously presented
by Hubel et al. ('75). In addition, the present
study shows that while the overall pattern of
retino-collicular projections are qualitatively
similar in monkey and cat (Graybiel, '75, '761,
there are notable differences both in terms of
the more prominent ipsilateral input to monkey superior colliculus (see also Hubel et al.,
'75) and the rather less distinct banding pattern seen in the colliculi of our monkeys.
Hubel et al. ('75) and Graybiel ('75, '76) both
report that the contralateral retino-collicular
projection forms a rather continuous band
throughout the more superficial level of the
upper gray in both the monkey and the cat.
Our findings differ from theirs slightly in that
we also find the dense contralateral projection
to be distributed in patches (figs. 2, 4 , 6 , 7) a t
least somewhat similar to those seen ipsilatera1 t o an eye injection. This finding is most
evident for regions lying between the optic
disc representation and the anterolateral
border of the colliculus. Along the medial and
posterolateral borders of the colliculus, and a t
deeper levels, the contralateral projection did
appear more continuous. In addition, regardless of depth beneath the surface of the col-
RETINO-COLLICULAR PROJECTIONS IN RHESUS MONKEYS
liculus, the contralateral projection always
becomes quite continuous near the posteromedial border of the superior colliculus; i.e., the
monocular segment.
In both the cat (Graybiel, '75, '76) and monkey (Hubel et al., '75) the ipsilateral projection is generally reported to occupy deeper
levels of the superficial gray than the projection arising from the contralateral eye. With
the exception of the superficial tier of the ipsilateral input seen anteromedially in some
monkeys, our findings are in agreement with
those previously reported. Such differences in
depth are especially evident for the projections from parts of the binocular visual field
beyond the central 10". Within the central
visual field representation we find relatively
little ipsilateral input, even though the contralateral projection is quite definite.
The rather striking topographical pattern
of retinocollicular projections in both cat and
monkey has led to the suggestion that right
and left eye inputs to the superior colliculus
may be segregated in a fashion similar to that
seen in layer IV of the monkey visual cortex
(Hubel et al., '68, '69, '72; Wiesel et al., '74;
LeVay et al., '75; and others). In the monkey
superior colliculus, the projections from the
two eyes are segregated in many areas, including the optic disc representation and the monocular segment. In addition, there are many
parts of the colliculus that clearly receive a
dense retinal input from both eyes. While both
types of input; i.e., overlapping and segregated, are organized in a band-like pattern
(we have refrained from calling these bands
columns since the colliculus is not organized
in a columner fashion equivalent to that seen
in cortex), this pattern is by no means as distinct or as regular as the ocular dominance
columns seen in visual cortex. In addition, our
present findings suggest that there may be
considerably more overlap between retinal
projections in the colliculus, especially if one
takes into account the fact that the areas containing sparse label are not illustrated in the
reconstructions. If such label represents a less
dense retinal projection (rather than label
contained in fibers-of-passage), then one is
faced with a more common situation where
the density of inputs from the two eyes varies
reciprocally as one moves across the part of
the colliculus on which the binocular visual
field is represented. One additional observation that would suggest this to be the case is
that the optic disc representation contralat-
601
era1 to the injected eye was always free of label, even when light label could be seen between dense patches of label in nearby parts of
the colliculus. Since there is no reason to
believe that fibers-of-passage would avoid the
optic disc representation, it seems likely that
any light label not contained in axon terminals would be as noticeable here as in other
nearby parts of the colliculus. With this in
mind, it is interesting to relate the pattern of
retinocollicular input seen here in adult animals t o the pattern of cortical ocular dominance columns seen in newborn monkeys
(Hubel et al., '77). During the first few weeks
of a monkey's postnatal life, there is a progressive segregation of ocular input to layer IV of
the visual cortex. Initially, the input from the
two eyes is largely overlapping. As the monkey's visual system matures more and more
geniculo-cortical afferents become confined to
their appropriate ocular dominance column
until finally the inputs from the two eyes are
totally segregated. Although it must be considered speculative at best, it is interesting to
hypothesize that the segregation of input to
the cortex and colliculus occurs in much the
same way except that the segregation of retino-collicular afferents has not reached the
level of development seen in the monkey
visual cortex; actually being more similar to
that seen in the cat visual cortex (Shatz et al.,
'77; LeVay et al., '78).
The anatomical findings reported here coincide well with previous physiological recordings made in monkey superior colliculus
(Cynader and Berman, '72; Schiller et al., '74).
Given the distribution of retino-collicular projections described here it is quite easy to understand how most superior colliculus cells
could be binocularly driven; although, given
the pattern of overlap in retinal projections
seen in our monkeys, one might expect t o more
often find areas where one eye is dominant
over the other (Hubel et al., '75), especially in
the parts of the colliculus near the optic disc
representation. In the antero-lateral third of
the superior colliculus the retinal projection,
as demonstrated by the autoradiographic
technique, is light. However, visually driven
cells have been recorded in this region, even
after the visual cortex has been cooled or removed (Schiller et al., '74). It is conceivable
that the autoradiographic technique does not
demonstrate all of the retinal projections to
this region, possibly missing some of the fine
fibered projections. Some support for this idea
602
J. G. POLLACK AND T. L. HICKEY
comes from the fact t h a t the animals showing
a retinal projection to this region were consistently those t h a t received t h e most radioactive amino acid. Given the balance of input to
this region from the two eyes, one might expect to record more contralaterally dominated
cells in a decorticate monkey. A slight tendency for this to occur is suggested in the histograms published by Schiller et al. (’74).
ACKNOWLEDGMENTS
We thank David Simmons and Gloria Avery
for their technical assistance and J. Gerard
for much of the computer programming. This
work was supported by N.I.H. Grants EY
01338 and EY 02159 and N.S.F. Grant BMS
74-23658 to T.L.H. Doctor Pollack was supported by N.I.H. Postdoctoral Fellowship F 32
EY 05112.
LITERATURE CITED
Brouwer, B., and W. P. C. Zeeman 1926 The projection of
the retina in the primary optic neurons in monkeys. J.
Physiol., 49: 1-35.
Bunt, A. H., A. E. Hendrickson, J. S. Lund, R. D. Lund and
A. F. Fuchs 1975 Monkey retinal ganglion cells: Morphometric analysis and tracing of axonal projections, with a
consideration of the peroxidase technique. J. Comp.
Neur., 164: 265-286.
Cynader, M., and N. Berman 1972 Receptive-fieldorganization of monkey superior colliculus. J. Neurophysiol., 35:
187-201.
Graybiel, A. M. 1975 Anatomical organization of retinotectal afferents in the cat: An autoradiographic study.
Brain Res., 96: 1-23.
1976 Evidence for banding of the cat’s ipsilatera1 retinotectal connection. Brain Res., 114: 318-327.
Hendrickson, A. E., M. E. Wilson and M. J. Toyne 1970 The
distribution of optic nerve fibers in Macaca mulatta.
Brain Res., 23: 425-427.
Hubel, D. H., S. LeVay and T. N. Wiesel 1975 Mode of termination of retinotectal fibers in macaque monkey: An
autoradiographic study. Brain Res., 96: 25-40.
Hubel, D. H., and T. N. Wiesel 1968 Receptive fields and
functional architecture of monkey striate cortex. J.
Physiol. (London), 195: 215-243.
- 1969 Anatomical demonstration of columns in
t h e monkey striate cortex. Nature (London), 221:
747-750.
1972 Laminar and columnar distribution of
geniculo-cortical fibers in the macaque monkey. J. Comp.
Neur., 146: 421-450.
Hubel, D. H., T. N. Wiesel and S. LeVay 1977 Plasticity of
ocular dominance columns in monkey striate cortex. Phil.
Trans. R. SOC.Lond. B., 278: 377-409.
LeVay, S.,D. H. Hubel and T. N. Wiesel 1975 The pattern of
ocular dominance columns in macaque visual cortex
revealed by a reduced silver stain. J. Comp. Neur., 159:
559-576.
LeVay, S., M. P. Stryker and C. J. Shatz 1978 Ocular dominance columns and their development in layer IV of the
cat’s visual cortex: A quantitative study. J. a m p . Neur.,
179: 223-244.
Malpeli, J. G., and F. H.Baker 1975 The representation of
the visual field in the lateral geniculate nucleus of
Macaca mulatta. J. Comp. Neur., 161: 569-594.
Schiller, P.H.,
M. Stryker, M. Cynader and N. Berman 1974
Response characteristics of single cells in the monkey superior colliculus following ablation or cooling of visual
cortex. J. Neurophysiol., 37: 181-194.
Shatz, C. J., S. H. Lindstrom and T. N. Wiesel 1977 The distribution of afferents representing the right and left eyes
in the cat’s visual cortex. Brain Res., 131: 103-116.
Wiesel, T.N., D. H. Hubel and D. K. Lam 1974 Autoradiographic demonstration of ocular-dominance columns i n
monkey striate cortex by means of transneuronal transport. Brain Res., 79: 273-279.
Wilson, M. E., and M. J. Toyne 1970 Retino-tectal and cortico-tectal projections in Macaca mulatta. Brain Res., 24:
395-406.
Wiitanen, J.T. 1969 Selective silver impregnation of degenerating axons and axon terminals in the central nervous system of the monkey (Macaca mulattai. Brain Res.,
14: 546-548.