* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Survey
Document related concepts
Transcript
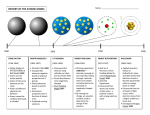
Fall 2015 Chapters 2 & 3 Atoms, Atomic Structure and Electronic structure Atoms 1 Fall 2015 Atoms: The Greek Idea ~384 B.C.E., Aristotle: All matter is composed of four elements and all matter is continuous, not atomistic. Aristotle declared matter to be infinitely divisible. Atoms: The Greek Idea ~ 450 B.C.E., Leucippus and Democritus Atomos: The point at which matter can no longer be subdivided. 2 Fall 2015 Atomic Theory of Matter The theory that atoms are the fundamental building blocks of matter reemerged in the early 19th century, championed by John Dalton. 1803 Dalton’s Postulates 1. Each element is composed of extremely small particles called atoms. 3 Fall 2015 Dalton’s Postulates 2. Atoms of an element cannot be created, destroyed, broken into smaller parts or transformed into atoms of another element • The discovery of nuclear processes showed that it was possible to transform atoms from one element into atoms of another. But we don't consider processes that affect the nucleus to be chemical processes. The postulate is still useful. A slightly more restrictive wording is "Atoms cannot be created, destroyed, or transformed into other atoms in a chemical change". Dalton’s Postulates 2. Atoms of an element cannot be created, destroyed, broken into smaller parts or transformed into atoms of another element • The discovery of nuclear processes showed that it was possible to transform atoms from one element into atoms of another. But we don't consider processes that affect the nucleus to be chemical processes. The postulate is still useful. A slightly more restrictive wording is "Atoms cannot be created, destroyed, or transformed into other atoms in a chemical change". 4 Fall 2015 Dalton’s Postulates 3. All atoms of a given element are identical to one another in mass and other properties, but the atoms of one element are different from the atoms of all other elements. Actually most elements occur in nature as mixtures of two or more kind of atoms called isotopes that have slightly different masses but same nuclear charges. In modern atomic theory, the postulate has been amended to read: "Elements are characterized by the nuclear charge of their atoms". Dalton’s Postulates 3. All atoms of a given element are identical to one another in mass and other properties, but the atoms of one element are different from the atoms of all other elements. Actually most elements occur in nature as mixtures of two or more kinds of atoms called isotopes that have slightly different masses but same nuclear charges. In modern atomic theory, the postulate has been amended to read: "Elements are characterized by the nuclear charge of their atoms". 5 Fall 2015 Dalton’s Postulates 4. When elements react, their atoms combine in simple, wholenumber ratios. Dalton’s Postulates 5.When elements react, their atoms sometimes combine in more than one simple, whole-number ratio. Nitrogen and oxygen combine to produce more than one product 6 Fall 2015 Law of Constant Composition Joseph Proust (1754–1826) • Also known as the law of definite proportions. • The elemental composition of a pure substance never varies. 1799 Regardless of the source, copper carbonate has the same composition. Malachite (mineral) Cu(OH)2CO3 7 Fall 2015 The Berzelius experiment illustrates the Law of Definite Proportions. Law of Conservation of Mass The total mass of substances present at the end of a chemical process is the same as the mass of substances present before the process took place. 1785 8 Fall 2015 Law of Conservation of Mass Law of Multiple Proportions “If two elements form more than one compound between them, then the ratios of the masses of the second element which combine with a fixed mass of the first element will be ratios of small whole numbers.” Or… ”If two elements form 2 different compounds, the mass ratio of the elements in one compound is related to the mass ratio in the other by a small whole number”. 1804 9 Fall 2015 Atomic structure Electricity and the Atom Electrolyte: A compound that conducts electricity when molten or dissolved in water. Electrodes: Carbon rods or metallic strips that carry electrical current. 10 Fall 2015 E Electrolysis Anode: A positive electrode. Cathode: A negative electrode. 1800: 1807: A. Volta invents the electrochemical cell H. Davy carries out the electrolysis of potassium hydroxyde Ions Ion: An atom or group of atoms with a charge. Anion: A negative ion. Cation: A positive ion. 11 Fall 2015 Discovery of the Electron • Streams of negatively charged particles were found to exit from cathode tubes. • J. J. Thomson is credited with their discovery (1897). 1897 Discovery of the Electron 1897, Joseph John Thomson: Determined the charge:mass ratio of cathode rays (discovered electrons). Charge/mass = 1.76 × 108 C/g 12 Fall 2015 The Electron Thomson used a magnetic field to bend the cathode rays. The amount the cathode ray bent from the straight line using the magnetic field allowed Thomson to calculate the e/m ratio= 1.76 108 coulombs/g. CRT TV (Cathode Ray Tube) 13 Fall 2015 CRT TV (Cathode Ray Tube) Refresh rate: 60-100 Hz Sir William Thomson (1824-1907) J. J. Thomson (1856-1940) British mathematical physicist British physicist 1892: Knighted by Queen Victoria. Titled « Baron Kelvin of Largs » (Lord Kelvin) 14 Fall 2015 Goldstein’s Experiment: Positive Particles 1886, Goldstein: Observed positive rays using a perforated cathode. 1886 Millikan's discovery 1913 15 Fall 2015 Millikan's discovery Robert Millikan determined the charge on the electron to be -1.60 10- 19 coulombs. For convenience, electronic charge is expressed as a multiple of that charge rather than coulombs. Thus the charge of the electronic charge is 1. Millikan's discovery 1) The drop is allowed to fall and its terminal velocity v1 in the absence of an electric field is calculated. The drag force (ie friction force/air resistance) acting on the drop, Fd, is terminal velocity of the falling drop radius of the drop viscosity of the air (eta) 16 Fall 2015 Millikan's discovery W m g d V g 2) For a perfectly spherical droplet the apparent weight, W, can be written as density of air radius of the drop gravity acceleration constant density of the oil Millikan's discovery 3) At terminal velocity the oil drop is not accelerating. This implies W F d 4 3 r g air 6rv1 3 r 9v1 2 g air 17 Fall 2015 Millikan's discovery 4) Now the electrical field is turned on, and the electric force on the drop, FE, is FE qE electrical field charge on the oil drop E For parallel plates, So: FE q V d V d potential difference distance between the plates Millikan's discovery 5) By adjusting V until the oil drop remains steady, we get FE W q V 4 3 r g air d 3 4 d q r 3 g air 3 V charge on the oil drop q was found to be a multiple of -1.60 10- 19 coulombs. 18 Fall 2015 Electron mass The electron mass was determined indirectly to be m= 9.10938 x 10-28g. X-Rays 1895, Wilhem Roentgen: Using a cathode ray tube, Roentgen discovered X-rays. shotgun pellets visible without surgery 3/38 1895 19 Fall 2015 Radioactivity • The spontaneous emission of radiation by a radioactive atom. • First observed by Henri Becquerel (1896) • Also studied by Marie and Pierre Curie. Radioactivity • Three types of radiation were discovered by Ernest Rutherford: particles particles rays 20 Fall 2015 Radioactivity Model of the atom J. J. Thomson who discovered the electron, proposed the “plum pudding model” of the atom 21 Fall 2015 Rutherford Gold Foil Experiment Using an apparatus similar to that shown below, Ernest Rutherford discovered the atomic nucleus. Rutherford Gold Foil Experiment 22 Fall 2015 The nuclear atom • Rutherford postulated a very small, dense nucleus with the electrons around the outside of the atom. • Most of the volume of the atom is empty space. 23 Fall 2015 Other subatomic particles • Protons were discovered by Rutherford (1919). • Neutrons were discovered by James Chadwick in (1932). Subatomic particles • Protons and electrons are the only particles that have a charge. • Protons and neutrons have essentially the same mass. • The mass of an electron is so small we ignore it. • 1 amu = 1.66054 x 10–24 g. 24 Fall 2015 Symbols of elements Elements are symbolized by one or two letters. Atomic Number All atoms of the same element have the same number of protons: The atomic number (Z) 25 Fall 2015 Atomic Mass The mass of an atom in atomic mass units (amu) is the total number of protons and neutrons in the atom. Isotopes • Atoms of the same element with different masses. • Isotopes have different numbers of neutrons. 11 6C 12 6C 13 6C 14 6C 26 Fall 2015 Isotopes of Hydrogen Electronic structure 27 Fall 2015 Quantum theory Branch of physics which deals with physical phenomena at microscopic scales. Quantum theory is the theoretical basis of modern physics that explains the nature and behavior of matter and energy on the atomic and subatomic level. It provides a mathematical description of much of the behavior and interactions of energy and matter. The wave nature of light • The electronic structure of an atom refers to the arrangement of electrons. • Interaction of light (electromagnetic radiation) with matter has provided us with a lot of information about the electronic structure of atoms. • Visible light is a form of electromagnetic radiation 28 Fall 2015 • All waves have a characteristic wavelength, l(lambda), amplitude, A and frequency n. • The speed of a wave is given by its frequency (hertz) multiplied by its wavelength. • For light, speed is c = n l Electromagnetic radiation moves through a vacuum with a speed of 3.00 x 108 m/s. Modern atomic theory arose out of studies of the interaction of radiation with matter. 29 Fall 2015 • The electromagnetic spectrum is a display of the various types of electromagnetic radiation arranged in order of increasing wavelength. • Example: visible radiation has wavelengths between 400 nm (violet) and 750 nm (red). 30 Fall 2015 Line Spectra and Bohr Model • Radiation composed of only one wavelength is called monochromatic. • Radiation that spans a whole array of different wavelengths is called continuous. • When radiation from a light source, such as a light bulb, is separated into its different wavelength components, a spectrum is produced. Continuous Spectrum White light can be separated into a continuous spectrum of colors. 31 Fall 2015 A rainbow is a continuous spectrum of light produced by the dispersal of sunlight by raindrops Line Spectrum 32 Fall 2015 Line Spectra Of Na and H Electron Arrangement: The Bohr Model Flame tests: Different elements give different colors to a flame. 33 Fall 2015 Bohr’s Model • Bohr noted the line spectra of certain elements and assumed the electrons were confined to specific energy states. These were called orbits. Bohr’s Model • Bohr’s model is based on three postulates: 1. Only orbits of specific radii, corresponding to certain definite energies, are permitted for electrons in an atom. 2. An electron in a permitted orbit has a specific energy and is in an "allowed" energy state. 3. Energy is only emitted or absorbed by an electron as it moves from one allowed energy state to another. (The energy is gained or lost as a photon). 34 Fall 2015 Energy Level in Hydrogen Atom Limitations of the Bohr Model • The Bohr Model has several limitations: • It cannot explain the spectra of atoms other than hydrogen. • Electrons do not move about the nucleus in circular orbits. However, the model introduces two important ideas: • The energy of an electron is quantized: electrons exist only in certain energy levels described by quantum numbers. • Energy gain or loss is involved in moving an electron from one energy level to another. 35 Fall 2015 Quantum Mechanics and Atomic Orbitals • Schrödinger proposed an equation containing both wave and particle terms. Solving the equation leads to wave functions • The wave function describes the electron’s matter wave. • The square of the wave function, 2, gives the probability of finding the electron. That is, 2 gives the electron density for the atom. • 2 is called the probability density. Electron density A region of high electron density is one where there is a high probability of finding an electron. 36 Fall 2015 • A collection of orbitals with the same value of n is called an electron shell. • A shell is made of one or more subshells. • Each subshell is designated by a number and a letter. Representations of Orbitals • All s orbitals are spherical. • As n increases, the s orbitals get larger. 37 Fall 2015 s Orbitals p Orbitals 38 Fall 2015 d Orbitals f Orbitals • When n is equal to 4 or larger, there are seven f orbitals for which l=3. • The shape of f orbitals are very complicated than those of d orbitals 39 Fall 2015 Many-Electron Atoms Orbitals and Their Energies • In a many-electron atom, for a given value of n, the energy of an orbital increases with increasing value of l (2s and 2p). • Orbitals of the same energy are said to be degenerate. Ordering of Orbital energy levels 40 Fall 2015 Electron Configurations • Electron configurations tell us how the electrons are distributed among the various orbitals of an atom. • The most stable configuration, or ground state, is that in which the electrons are in the lowest possible energy state. Assigning Electronic Configuration of a given atom • The following sequence is used: • 1s,2s,2p,3s,3p,4s,3d,4p,5s,4d,5p,6s,4f, 5d, 6p,7s,5f,6d..... • You begin with the first orbital, 1s, and add electrons until the maximum number for that orbital is reached, 41 Fall 2015 Assigning Electronic Configuration of a given atom • 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 4f 5d 6p 7s 5f 6d 7p Energy ordering rule Klechkowski’s rule Madelung’s rule 42 Fall 2015 Electron Configuration of Lighter Elements Transition Metals • After Ar the d orbitals begin to fill. • After the 3d orbitals are full the 4p orbitals begin to fill. • The ten elements between Sc and Zn are called the transition metals, or transition elements. 43 Fall 2015 Transition Metals Lanthanide and Actinide Elements • The 15 elements corresponding to the filling of 4f orbitals are called lanthanide elements (or rare earth elements). • The 15 elements corresponding to the filling of 5f orbitals are called actinide elements. • Most actinides are not found in nature (they are synthesized). 44 Fall 2015 Electron Configurations and the Periodic Table • The periodic table can be used as a guide for electron configurations. • The period number is the value of n. • Groups 1A and 2A have their s orbitals being filled. • Groups 3A – 8A have their p orbitals being filled. • The s-block and p-block of the periodic table contain the representative, or main-group, elements. • Transition metals have their d orbitals being filled. • The lanthanides and actinides have their f orbitals being filled. • The actinides and lanthanide elements are collectively referred to as the f-block metals. • Note that the 3d orbitals fill after the 4s orbital. Similarly, the 4f orbitals fill after the 5d orbitals. 45 Fall 2015 Electron Configuration of lanthanide • La [Xe] 6s2 5d1 4f 0 Lu [Xe] 6s2 5d1 4f 14 46 Fall 2015 The Periodic Table Electron Configurations and the Periodic Table The periodic table is considered by many to be the most predictive tool in all of chemistry. It is composed of vertical columns called groups or families and horizontal rows called periods. 47 Fall 2015 Electron Configurations and the Periodic Table Groups (families): Vertical columns in the periodic table. Groups contain elements with similar chemical properties. Periods: Horizontal rows in the periodic table. Elements in a period demonstrate a range of properties from metallic (on the left) to nonmetallic (on the right). Electron Configurations and the Periodic Table Valence electrons: Valence electrons are the electrons in the outermost principle energy level of an atom. These are the electrons that are gained, lost, or shared in a chemical reaction. Elements in a group or family have the same number of valence electrons. 48 Fall 2015 Electron Configurations and the Periodic Table Some groups in the periodic table have special names: • Alkali Metals: Group 1A – Valence electron configuration: ns1 • Alkaline Earth Metals: Group 2A – Valence electron configuration: ns2 • Halogens: Group 7A – Valence electron configuration: ns2np5 • Noble Gases: Group 8A – Valence electron configuration: ns2np6 Electron Configurations and the Periodic Table • Metals, Nonmetals, and Metalloids: – Metals • Metallic luster, conduct heat and electricity, malleable, and ductile. Examples are sodium and copper. – Nonmetals • Dull luster, nonconductors, and brittle. Examples are sulfur and bromine. – Metalloids • Demonstrate properties of both metals and nonmetals. Examples are silicon and arsenic. 3/98 49 Fall 2015 Electron Configurations and the Periodic Table 50