* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download New Approaches to Evaluate Mechanical Dyssynchrony г Potential
Coronary artery disease wikipedia , lookup
Heart failure wikipedia , lookup
Electrocardiography wikipedia , lookup
Cardiac surgery wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Echocardiography wikipedia , lookup
Myocardial infarction wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Ventricular fibrillation wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
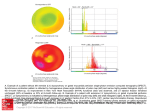
New Approaches to Evaluate Dyssynchrony Review Acta Cardiol Sin 2010;26:142-50 New Approaches to Evaluate Mechanical Dyssynchrony - Potential Usefulness in Predicting Response to Cardiac Resynchronization Therapy Chun-Li Wang,1,2 Chia-Tung Wu,1 Yung-Hsin Yeh,1 Lung-Sheng Wu1 and Chi-Tai Kuo1,2 Cardiac resynchronization therapy (CRT) is an established therapy for patients with advanced heart failure and wide QRS intervals. Numerous single-center studies indicated that the assessment of mechanical dyssynchrony helps predict response to CRT. However, two multi-center studies disprove the idea that the conventional assessment of mechanical dyssynchrony is useful for CRT patient selection. The purpose of this review is to provide an overview of the conventional methods as well as three novel approaches (cross-correlation analysis, Fourier analysis of strain uniformity, and discoordination analysis) for dyssynchrony assessments and their potential usefulness in predicting response to CRT. Key Words: Cardiac resynchronization therapy · Dyssynchrony · Echocardiography INTRODUCTION Mechanical dyssynchrony assessment: conventional steps and its limitations Mechanical dyssynchrony, the difference in regional contraction timing, is usually a consequence of electrical dyssynchrony due to ventricular conduction delay or right ventricular pacing. 8-15 Data from single-center studies suggests that numerous parameters of mechanical dyssynchrony can improve patient selection for CRT on the top of a wide QRS duration.8-10,12-14 These parameters include the septal-to-posteior wall motion delay on M-mode echocardiography (> 130 ms), anteroseptalto-posterior wall delay using speckle tracking radial strain (> 130 ms) and several indices based on tissue Doppler imaging (TDI), such as septal to lateral delay (SLD) in apical 4-chamber view (> 60 ms), maximum difference in opposing wall delay (MD) in apical 4chamber or long-axis views (> 65 ms) and the standard deviation of 12 sites (TsSD) using longitudinal tissue Doppler velocities (> 34 ms).8-10,12,16-19 However, recent two multi-center prospective studies disprove the idea that the assessment of mechanical dyssynchrony is useful for CRT patient selection. The Cardiac resynchronization therapy (CRT) is an established treatment for patients with advanced heart failure (HF) and wide QRS intervals (> 0.12 sec).1,2 CRT improves cardiac function and clinical symptoms and reduces mortality. 3-8 However, despite its overall efficacy, 30% to 40% patients receiving CRT do not benefit from the therapy, and one concern is that patient selection, which has been based on a wide QRS duration, inadequately identifies mechanical dyssynchrony.8-12 It has been proposed that mechanical dyssynchrony rather than electrical dyssynchrony better predicts response to CRT.8-10,12-14 Received: October 19, 2009 Accepted: November 24, 2009 1 First Division of Cardiovascular Department, Chang Gung Memorial Hospital, Linkou Branch; 2College of Medicine, Chang Gung University, Taoyuan, Taiwan. Address correspondence and reprint requests to: Dr. Chi-Tai Kuo, First Division of Cardiovascular Department, Chang Gung Memorial Hospital, Linkou Branch, Taoyuan, Taiwan. Tel: 886-3-328-1200, ext. 8162; Fax: 886-3-327-1192; E-mail: [email protected] Acta Cardiol Sin 2010;26:142-50 142 New Approaches to Evaluate Dyssynchrony the adverse consequences of asynchronous activation by stimulating the right ventricle and left ventricle simultaneously or pacing the left ventricle only. The conventional steps for quantifying mechanical dyssynchrony are as follows. First, curves to depict the motion or deformation of ³ 2 segments of the left ventricle (velocity, strain or strain rate) are determined from TDI or speckle tracking echocardiography. Second, the time from a reference point on electrocardiogram to a peak of velocity or strain is measured for each curve. Finally, mechanical dyssynchrony is quantified from the dispersion of the time-to-peak values (the difference or the standard deviation). Although fast and easy to measure, time-to-peak analysis that uses only a single data point of the velocity or strain curve is methodologically simplistic and likely susceptible to noise and technical factors. Recent studies including the PROSPECT trial have shown that time-to-peak analysis is highly observer-dependent and poorly reproducible, even in normal controls.20,27 Peak systolic velocities from TDI are often difficult to locate in cases of multiple peaks, a flat velocity contour, marked beat-to-beat variations and no positive velocity during systole.28 Another issue is that TDI measures motion relative to the transducer, whether active or passive. The failing heart may show a rocking movement that is difficult to interpret the data and that the measured value may not really represent asynchronous contraction.29 Most of mechanical dyssynchrony analyses only assess the time differences of mechanical activity in different regions and do not consider the magnitude of regional contraction. Therefore, such analysis is unable to clarify whether the measured value truly reflects a temporal delay in mechanical contraction due to asynchronous activation or the heterogeneity of LV contraction in the wall. The former is a likely target for CRT, whereas for the latter, the role of CRT is not clear.25 Predictors of Response to CRT (PROSPECT) trial prospectively evaluated the value of 12 echocardiographic metrics of mechanical dyssynchrony to predict response to CRT, based on both conventional and TDI-based methods, in 426 patients with HF and a standard indication for CRT from 53 medical centers.20 Given the modest sensitivity and specificity in this trial, no echocardiographic measure of dyssynchrony could be recommended to improve patient selection for CRT beyond current guidelines. Likewise, the Resynchornization Therapy in Patients with HF and Narrow QRS (RethinQ) trial showed that patients with a QRS duration < 130 ms and evidence of mechanical dyssynchrony, primarily based on TDI measures, did not benefit from CRT.21 In view of the two negative studies of mechanical dyssynchrony, we are faced with a paradox. On one side, numerous single-center studies indicated that the assessment of mechanical dyssynchrony helps predict response to CRT. On the other side, randomized multi-center trials showed contradictory results. Although many studies have been done to quantify mechanical dyssynchrony in hopes of refining patient selection, there is still no ideal dyssynchrony parameter that can be used to recommend whether a patient should undergo CRT. Many experts believe that techniques to quantify mechanical dyssynchrony need to be refined, not abandoned, and will play a role in selecting patients for CRT in the future.22-26 To realize the limitations of mechanical dyssynchrony, it is necessary to know why mechanical dyssynchrony might occur and how we measure it. In normal subjects, the left ventricle is activated almost simultaneously through the rapid conduction system, and this is followed by synchronous contraction. Patients who have left bundle branch block or other ventricular conduction disturbances have a markedly different activation sequence. In patients with left bundle branch block, the inter-ventricular septum and right ventricle are activated early, whereas the posterior and lateral left ventricular (LV) walls are activated late. The time difference in activation leads to early septal and late posterior-lateral contraction. Early septal contraction causes posterior-lateral thinning or stretching, followed by late posterior-lateral contraction causing septal thinning or stretching. This asynchronous contraction generates heterogeneous strain and stress in the left ventricle and results in inefficient LV performance. CRT can reverse Cross-correlation analysis of dyssynchrony Fornwalt et al. developed a new parameter, crosscorrelation delay (XCD), to measure dyssynchrony throughout the cardiac cycles.30,31 XCD uses a signal processing method called a cross-correlation analysis that utilizes all velocity data from 3 consecutive beats. The analysis is computed between two tissue velocity curves by shifting one curve in relation to the other along the 143 Acta Cardiol Sin 2010;26:142-50 Chun-Li Wang et al. ing mechanical dyssynchrony by XCD or XCA may be useful for expanding the benefits of CRT to potential subjects who do not fulfill the current CRT criteria. time axis and computing the correlation coefficient between the two curves for each time shift. A cross-correlation coefficient value of 1 indicates that the two curves are completely synchronous in time, whereas a value of 0 indicates that there is no correlation between the two curves. The time shift that results in the maximum correlation coefficient is the temporal delay (Figure 1). The advantage of XCD is that the analysis does not require identification of ejection phase or manual selection of peak systolic velocities. Fornwalt et al. compared the ability of XCD with the conventional dyssynchrony parameters SLD, MD and TsSD to discriminate between normal controls and CRT responders.31 XCD performed better than conventional parameters in discriminating responders from normal controls. XCD was also the only one that showed a decrease after CRT in responders. However, it is important to note that the temporal delay derived from the crosscorrelation analysis may be inaccurate when the correlation is weak (e.g., correlation coefficient < 0.5).31 This concern may become important when using cross-correlation analysis to distinguish between CRT responders and non-responders. In a recent study, Olsen et al showed that XCD was unable to distinguish between non-responders and responders.32 They used acceleration data instead of velocity data for cross-correlation analysis. Myocardial acceleration was computed from the differentiation of velocity data and was tentatively more close to contractility than velocity. The investigators evaluated the ability of cross-correlation analysis of systolic myocardial acceleration (XCA) with SLD and TsSD to discriminate between control groups (with narrow QRS) and CRT responders and between responders and non-responders.32 Similar to XCD, XCA discriminated CRT responders from control groups with a significantly higher area under curve (AUC) than SLD and TsSD (0.95 vs. 0.59 and 0.75, respectively). XCA also discriminated responders from non-responders (AUC = 0.66) but not significantly better than SLD (AUC = 0.55) and TsSD (AUC = 0.58). With XCA, dyssynchrony in control subjects was found to be rare, whereas with conventional measures, dyssynchrony was found in > 30 % of control subjects. This finding suggests that conventional dyssynchrony metrics are less specific and overestimate the prevalence of mechanical dyssynchrony. Better specificity at detectActa Cardiol Sin 2010;26:142-50 Fourier analysis for strain uniformity Mechanical dyssynchrony can be quantified by the Fourier decomposition of myocardial strain. 33-35 The Fourier analysis of each component of the strain is determined over time and space. The zero-order component S0 of the Fourier decomposition is constant and represents perfectly synchronous contraction, whereas the first-order component, S1, is sinusoidal and represents completely asynchronous contraction. The temporal uniformity of strain (TUS) or circumferential uniformity ratio estimate (CURE) index is calculated as the ratio of the sum of the synchronous segments (S0) and the sum of the both S1 and S0 over time.33-35 For a perfectly synchronous motion, TUS or CURE index provides a value of 1, whereas for a completely asynchronous motion, it is equal to 0 (Figure 2). Byrne et al. used the magnetic resonance imaging and CURE index to compare mechanical dyssynchrony and the impact of CRT on dyssynchrony between failing hearts with a right bundle branch block versus left bundle branch block. 33 They found that mechanical dyssynchrony was less in right bundle branch block and CRT had less effect on failing hearts with right bundle branch block than those with left bundle branch block. Bertola et al. calculated the TUS index from speckletracking strain data.35 They found that radial TUS predicted response to CRT (AUC = 0.65), whereas dyssynchrony measure (TsSD strain ) did not (AUC = 0.54) and was the only one that predicted LV ejection fraction improvement after CRT. Fourier analysis of strain uniformity is a more sophisticated quantitative method as compared to conventional dyssynchrony assessment. The TUS (or CURE) index takes a geographic dispersion of strain into account and may differentiate temporal delays in contraction due to heterogeneity of a failing heart from temporal delays due to asynchronous activation. However, the analysis assumes a sine wave spatial variation in strain which may be not true in some patients. In addition, TUS are not very reproducible. From the Bland-Altman plots shown in the study of Bertola et al., the 95% limits of agreement are almost the same as the mean values of TUS.22,35 144 New Approaches to Evaluate Dyssynchrony A B C Figure 1. Myocardial velocity traces and cross-correlation analysis of myocardial velocity. Arrows mark peak systolic velocities in velocity traces and temporal delay (TD) as well as maximum cross-correlation coefficients (XCC) in cross-correlation analysis plots. (A) Despite a large difference in time-to-peak systolic velocity (151 ms) in a normal subject, cross-correlation analysis reveals a high degree of synchrony: TD = 9 ms and XCC = 0.88. (B) Baseline date of a CRT responder. Velocity analysis shows significantly delayed peak of the posterior wall (98 ms). Cross-correlation analysis reveals a significant delay (TD = 162 ms). (C) Six-month follow-up data of a CRT responder. Velocity analysis shows a delayed peak of the posterior wall (63 ms) but cross-correlation analysis indicates a high degree of synchrony: TD = 0 ms and XCC = 0.9. AVO, aortic valve opening; AVC, aortic valve closure. 145 Acta Cardiol Sin 2010;26:142-50 Chun-Li Wang et al. A B C Figure 2. Three-dimensional plot of radial strain in a normal subject (A) and a CRT responder before (B) and 6 months after CRT (C) arranged according to time and ventricular location (left panels); temporal uniformity of strain (TUS) in the same subject evaluated by means of Fourier analysis (right panels). (A) TUS was 0.998, suggesting synchronous thickening around the ventricle. (B) Baseline TUS was 0.652, suggesting dyssynchrony. (C) TUS improved from 0.652 to 0.959, indicating resynchronization after 6 months of CRT. tients with a wide QRS duration.22,25 A more comprehensive assessment of regional myocardial mechanics may be achieved from indices describing mechanical discoordination, i.e. the amount and distribution of lengthening/shortening or thinning/thickening within the LV Analysis of mechanical discoordination The poor performance of mechanical dyssynchrony metrics in the multi-center studies may be that time differences in onset or peak velocity or strain only partially reveal the mechanical disturbance of left ventricle in paActa Cardiol Sin 2010;26:142-50 146 New Approaches to Evaluate Dyssynchrony wall. 36 Kirn et al. developed a novel discoordination measure, called internal stretch fraction (ISF), to quantify the relative circumferential lengthening (internal stretch) versus circumferential shortening during the ejection phase by tagging magnetic resonance imaging.36 The amount of stretch (or shortening) was calculated as the product of the number of regions with stretch (or shortening) and their average amount of stretch (or shortening), integrated over the ejection phase (Figure 3). They compared the new index with dyssynchrony metrics and found that only ISF could differentiate responders from non-responders. It will be highly valuable to quantify ISF by echocardiography that makes its application and post-CRT A B C Figure 3. Radial strain rate plots and discoordination analysis (ISF) in a normal subject (A) and a CRT responder before (B) and 6 months after CRT (C). Tracings of strain rate of 6 mid-ventricular segments are represented as gray lines. The time course of the average thickening [ep(t)] and average thinning [en(t)] are represented as blue line and red line, respectively. The amount of myocardial thickening (ep) and thinning (en) during the ejection phase are represented with the area below curve ep(t) and above curve en(t), respectively. Internal stretch fraction (ISF) was calculated as -(en/ep) ´ 100%. (A) ISF was 0, suggesting no discoordination. (B) Baseline ISF was 118%, suggesting marked discoordination. (C) ISF decreased from 118% to 10%, indicating recoordination after 6 months of CRT. AVO, aortic valve opening; AVC, aortic valve closure. 147 Acta Cardiol Sin 2010;26:142-50 Chun-Li Wang et al. times with potentially different heart rates and loading conditions from when the mid-ventricular images were acquired, so that the marking of ejection phase adds a source of potential error to the quantification of ISF. In the above sections, we describe the commonly used conventional dyssynchrony parameters and three new approaches for assessing mechanical dyssynchrony. Advantages and disadvantages of each echocardiographic method for the quantification of mechanical dyssynchrony are summarized in Table 1. Although the presence of LV dyssynchrony appears to play an important role in determining the response to CRT, many factors are associated with the outcome of CRT-treated patients. Comprehensive assessments of mechanical dyssynchrony, myocardial scar burden, the site of latest mechanical activation, and venous anatomy pre-operatively may help to identify those who will not derive any benefit or be potentially worsened.40,41 follow-up more practical and cheaper, and lessens the burden for patients with heart failure. From our initial experience, mechanical discoordination could be quantified using speckle-tracking echocardiography. 37 We found less discoordination in normal subjects and most CRT non-responders. An acute reduction in discoordination (recoordination) rather than resynchronization predicted LV reverse remodeling after CRT.37 ISF is conceptually related to internal flow fraction, which can be measured by conductance catheter or magnetic resonance imaging and has been shown to decrease significantly after CRT.38,39 When there are alternating regions of myocardial expansion and contraction, blood will be sequestered in the heart rather than ejected to the systemic circulation. The internal flow fraction or ISF measures the overall inefficiency of the heart by comparing the amount of effective contraction with the amount of internal energy wasting.36-39 The advantage of ISF over conventional metrics is that it takes into account regional dispersion of strain during the ejection period, rather than at a single time point in a strain signal. The limitation of ISF is its dependency on timing of aortic opening and closure. The timing must be collected at different CONCLUSION Whenever a novel method develops that will open a Table 1. Advantages and disadvantages of echocardiographic methods for the assessment of mechanical dyssynchrony Methods 17,18 Advantages Disadvantages M-mode No specific echocardiographic machine is needed Easy to perform Difficult to determine the timing of peak inward motion if the wall is akinetic or has complex motion A high degree of variability Color-coded tissue Doppler velocity8-10,16,19 High temporal resolution Allows simultaneous comparison of multiple segments Susceptible to translational motion or tethering effect A high degree of variability Radial strain by speckle tracking12,14 Less affected by translational motion and tethering effect Less variability Require specific software Less temporal resolution Cross-correlation analysis31,32 Nearly automated analysis Less variability Rare dyssynchrony detected in normal subjects The calculated time delay may be inaccurate if the velocity correlation is weak Fourier analysis of strain uniformity33-35 Nearly automated analysis Allows assessment of geographic strain dispersion Sophisticated method Based on the assumption of a sine wave variation in strain Discoordination analysis36,37 Nearly automated analysis Allows assessment of the amount of internal stretch (energy wasting) Requires identification of ejection phase Acta Cardiol Sin 2010;26:142-50 148 New Approaches to Evaluate Dyssynchrony new window into the heart function and disclose things that we have not appreciated previously. Advances in analysis and better understanding of dyssynchrony and discoordination will help us find an accurate, reliable and practical parameter for selecting CRT patients in the future. Until then, patient selection must use the parameter previously validated in landmark clinical studies: the QRS duration. 8. 9. 10. ACKNOWLEDGEMENT This study was supported by research grant CMRPG 381221 from the Chang Gung Memorial Hospial, Linkou branch, Taiwan. 11. 12. REFERENCES 13. 1. Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/ American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices): developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation 2008;117:e350-408. 2. Hunt SA, Abraham WT, Chin MH, et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation 2009;119:e391-479. 3. Cazeau S, Leclercq C, Lavergne T, et al. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med 2001;344:873-80. 4. Auricchio A, Stellbrink C, Sack S, et al. Long-term clinical effect of hemodynamically optimized cardiac resynchronization therapy in patients with heart failure and ventricular conduction delay. J Am Coll Cardiol 2002;39:2026-33. 5. Breithardt OA, Stellbrink C, Franke A, et al. Acute effects of cardiac resynchronization therapy on left ventricular Doppler indices in patients with congestive heart failure. Am Heart J 2002;143: 34-44. 6. Abraham WT. Cardiac resynchronization therapy for the management of chronic heart failure. Am Heart Hosp J 2003;1:55-61. 7. Breithardt OA, Stellbrink C, Herbots L, et al. Cardiac resynchronization therapy can reverse abnormal myocardial strain dis- 14. 15. 16. 17. 18. 19. 20. 21. 22. 149 tribution in patients with heart failure and left bundle branch block. J Am Coll Cardiol 2003;42:486-94. Bax JJ, Bleeker GB, Marwick TH, et al. Left ventricular dyssynchrony predicts response and prognosis after cardiac resynchronization therapy. J Am Coll Cardiol 2004;44:1834-40. Penicka M, Bartunek J, De Bruyne B, et al. Improvement of left ventricular function after cardiac resynchronization therapy is predicted by tissue Doppler imaging echocardiography. Circulation 2004;109:978-83. Yu CM, Fung JW, Zhang Q, et al. Tissue Doppler imaging is superior to strain rate imaging and postsystolic shortening on the prediction of reverse remodeling in both ischemic and nonischemic heart failure after cardiac resynchronization therapy. Circulation 2004;110:66-73. Hawkins NM, Petrie MC, Macdonald MR, et al. QRS duration alone to select patients for cardiac resynchronization therapy: flying in the face of the evidence? Eur Heart J 2006;27:3074-5. Suffoletto MS, Dohi K, Cannesson M, et al. Novel speckle-tracking radial strain from routine black-and-white echocardiographic images to quantify dyssynchrony and predict response to cardiac resynchronization therapy. Circulation 2006;113:960-8. Yu CM, Fung WH, Zhang Q, et al. Understanding nonresponders of cardiac resynchronization therapy -- current and future perspectives. J Cardiovasc Electrophysiol 2005;16:1117-24. Gorcsan J 3rd, Tanabe M, Bleeker GB, et al. Combined longitudinal and radial dyssynchrony predicts ventricular response after resynchronization therapy. J Am Coll Cardiol 2007;50: 1476-83. Wu CT, Kuo CT, Luqman N, et al. Three-dimensional automated contour detection for global systolic dyssynchrony index measurements. Acta Cardiol Sin 2008;24:136-43. Soliman OI, Theuns DA, Geleijnse ML, et al. Spectral pulsedwave tissue Doppler imaging lateral-to-septal delay fails to predict clinical or echocardiographic outcome after cardiac resynchronization therapy. Europace 2007;9:113-8. Pitzalis MV, Iacoviello M, Romito R, et al. Ventricular asynchrony predicts a better outcome in patients with chronic heart failure receiving cardiac resynchronization therapy. J Am Coll Cardiol 2005;45:65-9. Pitzalis MV, Iacoviello M, Romito R, et al. Cardiac resynchronization therapy tailored by echocardiographic evaluation of ventricular asynchrony. J Am Coll Cardiol 2002;40:1615-22. Yu CM, Fung WH, Lin H, et al. Predictors of left ventricular reverse remodeling after cardiac resynchronization therapy for heart failure secondary to idiopathic dilated or ischemic cardiomyopathy. Am J Cardiol 2003;91:684-8. Chung ES, Leon AR, Tavazzi L, et al. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation 2008;117: 2608-16. Beshai JF, Grimm RA, Nagueh SF, et al. Cardiac-resynchronization therapy in heart failure with narrow QRS complexes. N Engl J Med 2007;357:2461-71. Fornwalt BK, Delfino JG, Sprague WW, et al. It’s time for a paraActa Cardiol Sin 2010;26:142-50 Chun-Li Wang et al. 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. digm shift in the quantitative evaluation of left ventricular dyssynchrony. J Am Soc Echocardiogr 2009;22:672-6. Rademakers LM, de Boeck BW, Maessen JG, et al. Development of strategies for guiding cardiac resynchronization therapy. Heart Fail Clin 2008;4:333-45. Marwick TH. Hype and hope in the use of echocardiography for selection for cardiac resynchronization therapy: the Tower of Babel revisited. Circulation 2008;117:2573-6. Kass DA. An epidemic of dyssynchrony: but what does it mean? J Am Coll Cardiol 2008;51:12-7. Gorcsan J 3rd, Abraham T, Agler DA, et al. Echocardiography for cardiac resynchronization therapy: recommendations for performance and reporting - a report from the American Society of Echocardiography Dyssynchrony Writing Group endorsed by the Heart Rhythm Society. J Am Soc Echocardiogr 2008;21:191-213. Conca C, Faletra FF, Miyazaki C, et al. Echocardiographic parameters of mechanical synchrony in healthy individuals. Am J Cardiol 2009;103:136-42. Anderson LJ, Miyazaki C, Sutherland GR, et al. Patient selection and echocardiographic assessment of dyssynchrony in cardiac resynchronization therapy. Circulation 2008;117:2009-23. Marwick TH, Starling RC. The riddle of determining cardiac resynchronization therapy response a physiologic approach to dyssynchrony therapy. J Am Coll Cardiol 2008;52:1410-2. Delfino JG, Fornwalt BK, Eisner RL, et al. Cross-correlation delay to quantify myocardial dyssynchrony from phase contrast magnetic resonance (PCMR) velocity data. J Magn Reson Imaging 2008;28:1086-91. Fornwalt BK, Arita T, Bhasin M, et al. Cross-correlation quantification of dyssynchrony: a new method for quantifying the synchrony of contraction and relaxation in the heart. J Am Soc Echocardiogr 2007;20:1330-7. Olsen NT, Mogelvang R, Jons C, et al. Predicting response to cardiac resynchronization therapy with cross-correlation analysis of myocardial systolic acceleration: a new approach to echocardiographic dyssynchrony evaluation. J Am Soc Echocardiogr 2009; Acta Cardiol Sin 2010;26:142-50 22:657-64. 33. Byrne MJ, Helm RH, Daya S, et al. Diminished left ventricular dyssynchrony and impact of resynchronization in failing hearts with right versus left bundle branch block. J Am Coll Cardiol 2007;50:1484-90. 34. Helm RH, Leclercq C, Faris OP, et al. Cardiac dyssynchrony analysis using circumferential versus longitudinal strain: implications for assessing cardiac resynchronization. Circulation 2005;111:2760-7. 35. Bertola B, Rondano E, Sulis M, et al. Cardiac dyssynchrony quantitated by time-to-peak or temporal uniformity of strain at longitudinal, circumferential, and radial level: implications for resynchronization therapy. J Am Soc Echocardiogr 2009;22: 665-71. 36. Kirn B, Jansen A, Bracke F, et al. Mechanical discoordination rather than dyssynchrony predicts reverse remodeling upon cardiac resynchronization. Am J Physiol Heart Circ Physiol 2008; 295:H640-6. 37. Wang CL, Wu CT, Yeh YH, et al. Recoordination rather than resynchronization predicts reverse remodeling after cardiac resynchronization therapy. J Am Soc Echocardiogr 2010;23:611-20. 38. Fornwalt BK, Gonzales PC, Delfino JG, et al. Quantification of left ventricular internal flow from cardiac magnetic resonance images in patients with dyssynchronous heart failure. J Magn Reson Imaging 2008;28:375-81. 39. Steendijk P, Tulner SA, Schreuder JJ, et al. Quantification of left ventricular mechanical dyssynchrony by conductance catheter in heart failure patients. Am J Physiol Heart Circ Physiol 2004; 286:H723-30. 40. Nazar L., Wang CL, Sung RJ, Kuo CT. Emerging role of cardiac resynchronization therapy in heart failure. Acta Cardiol Sin 2008;24:1-14. 41. Sanderson JE. Echocardiography for cardiac resynchronization therapy selection. Fatally flawed or misjudged? J Am Coll Cardiol 2009;53:1960-4. 150