* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download The shoot apical meristem and development of vascular architecture1
Plant secondary metabolism wikipedia , lookup
Plant ecology wikipedia , lookup
Plant defense against herbivory wikipedia , lookup
Plant reproduction wikipedia , lookup
Plant physiology wikipedia , lookup
Plant stress measurement wikipedia , lookup
Venus flytrap wikipedia , lookup
Arabidopsis thaliana wikipedia , lookup
Evolutionary history of plants wikipedia , lookup
Plant morphology wikipedia , lookup
Perovskia atriplicifolia wikipedia , lookup
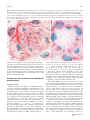
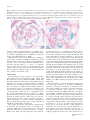
1660 REVIEW / SYNTHÈSE The shoot apical meristem and development of vascular architecture1 Nancy G. Dengler Abstract: The shoot apical meristem (SAM) functions to generate external architecture and internal tissue pattern as well as to maintain a self-perpetuating population of stem-cell-like cells. The internal three-dimensional architecture of the vascular system corresponds closely to the external arrangement of lateral organs, or phyllotaxis. This paper reviews this correspondence for dicotyledonous plants in general and in Arabidopsis thaliana (L.) Heynh., specifically. Analysis is partly based on the expression patterns of the class III homeodomain-leucine zipper transcription factor ARABIDOPSIS THALIANA HOMEOBOX GENE 8 (ATHB8), a marker of the procambial and preprocambial stages of vascular development, and on the anatomical criteria for recognizing vascular tissue pattern. The close correspondence between phyllotaxis and vascular pattern present in mature tissues arises at early stages of development, at least by the first plastochron of leaf primordium outgrowth. Current literature provides an integrative model in which local variation in auxin concentration regulates both primordium formation on the SAM and the first indications of a procambial prepattern in the position of primordium leaf trace as well as in the elaboration of leaf vein pattern. The prospects for extending this model to the development of the complex three-dimensional vascular architecture of the shoot are promising. Key words: ATHB8, auxin, phyllotaxis, ATPIN1, procambium, vascular development. Résumé : La fonction du méristème apical de la tige est de générer l’architecture externe et le patron histologique interne, ainsi que de maintenir une population de cellules de nature caulinaire par auto-perpétuation. L’architecture interne tridimensionnelle du système vasculaire correspond étroitement à l’arrangement externe des organes latéraux, ou phyllotaxie. L’auteur passe en revue cette correspondance chez les plantes dicotyles en général, et plus particulièrement chez l’Arabidopsis thaliana. L’analyse est partiellement basée sur l’expression des patrons de classe III du facteur de transcription de l’homéodomaine-leucine-zipper, ARABIDOPSIS THALIANA HOMEOBOX GENE 8 (ATHB8), un marqueur des stades cambial et procambial du développement vasculaire, ainsi que sur des critères anatomiques pour reconnaı̂tre le patron des tissus vasculaires. L’étroite correspondance entre la phyllotaxie et le patron des tissus vasculaires, dans les tissus matures, apparaı̂t à un stade précoce du développement, au moins au premier plastochron de l’apparition du primordium foliaire. La littérature courante présente un modèle intégrateur dans lequel la variation locale des teneurs en auxines règle à la fois la formation du primordium sur le méristème apical de la tige et les premières indications d’un pré-patron procambial, dans la position de la trace foliaire du primordium, ainsi que dans l’élaboration du patron vasculaire foliaire. La perspective d’étendre ce modèle de développement de l’architecture vasculaire tridimensionnelle complexe de la tige apparaı̂t prometteuse. Mots clés : ATHB8, auxine, phyllotaxie, ATP1N1, procambium, développement vasculaire. [Traduit par la Rédaction] Introduction The shoot apical meristem (SAM) functions to generate external architecture and internal tissue pattern as well as to maintain a self-perpetuating population of cells. Knowledge of the development and behavior of the apical meristems is Received 22 February 2006. Published on the NRC Research Press Web site at http://canjbot.nrc.ca on 6 February 2007. N.G. Dengler. Department of Botany, University of Toronto, Toronto, ON M5S 1A1, Canada (e-mail: [email protected]). 1This review is one of a selection of papers published on the Special Theme of Shoot Apical Meristems. Can. J. Bot. 84: 1660–1671 (2006) prerequisite for understanding plant development as well as the special properties of plants as organisms with an indeterminate body plan. Recently, attention has focused on the generation of external architecture, specifically the placement of lateral organs (e.g., Fleming 2005; Reinhardt 2005), and on the formation and maintenance of the population of stem-cell-like cells at the core of the SAM (e.g., Baurle and Laux 2003; Carles and Fletcher 2003). Less attention has been given to the generation of the pattern of dermal, ground, and vascular tissues within the shoot. While the protoderm (precursor of the dermal tissue system) is derived from the surface layer (L1) of the SAM simply by a restriction of division plane to anticlinal, the gradual emergence of vascular pattern from more homogeneous-appearing precur- doi:10.1139/B06-126 # 2006 NRC Canada Dengler sors derived from the L2 and deeper layers of the SAM is less well understood (Steeves and Sussex 1989). The procambium (vascular tissue precursor) becomes distinct from surrounding ground meristem (ground tissue precursor) by differential patterns of cellular vacuolation, division, and enlargement (Esau 1965a, 1965b). Procambial pattern can be recognized because the component cells are elongate in shape and less vacuolated than adjacent ground meristem and form continuous strands (Esau 1965a, 1965b; Nelson and Dengler 1997). As it emerges, the procambial system can be seen to form a complex, three-dimensional architecture within the shoot that is continuous with more mature parts of the vascular system. Moreover, the complex internal architecture of the vascular system corresponds closely to the external architecture of lateral organ arrangement, or phyllotaxis. The purpose of this review is to examine the close correspondence between phyllotaxis and primary vascular architecture. The literature on this subject extends back for almost 150 years, since botanists such as Nägeli (1858) and DeBary (1884) noted that the geometric array of developing and mature leaves gives an external clue to the internal arrangement of vascular bundles. In past decades, the causality of this correspondence has been hotly debated, but as emphasized by Esau (1965a, 1965b), the opposing views that either (i) new primordia induce the formation of the vascular bundles that supply them or (ii) acropetal development of vascular bundles induces the formation of primordia are oversimplifications, and it is more likely that both phyllotaxis and vascular architecture are determined by a common mechanism. Recent experimental and modeling studies have provided strong evidence for such a common mechanism (Fleming 2005; Reinhardt and Kuhlemeier 2002; Reinhardt et al. 2003; Reinhardt 2005; Smith et al. 2006; Jönsson et al. 2006). In this paper, I first review the expression pattern of ARABIDOPSIS THALIANA HOMEOBOX GENE8 (ATHB8), a putative marker of procambium, and its precursors within the SAM region. Second, I review primary vascular architecture of dicotyledonous shoots and its correspondence to phyllotaxis and then describe this correspondence for A. thaliana based on an analysis of procambium anatomy and the expression pattern of ATHB8 (based on the results of Kang et al. 2003). Third, I describe developmental aspects of procambium pattern, including the pattern of ATHB8 expression. Finally, I review a current model for regulation of both phyllotaxis and its corresponding vascular architecture by active modulation of local auxin concentration. ATHB8 as a marker of primary vascular development The ARABIDOPSIS THALIANA HOMEOBOX GENE8 (ATHB8) is one of five members of a family of class III homeodomain-leucine zipper (HD-Zip) transcription factors in the Arabidopsis genome that functions in the formation of meristems, in the dorsiventral patterning of lateral organs, and in the patterning and differentiation of vascular tissues (McConnell et al. 2001; Emery et al. 2003; Floyd and Bowman 2004). Four members of the family (REVOLUTA, PHABULOSA, PHAVOLUTA, and ATHB15/CO- 1661 RONA) are expressed in the SAM, adaxial domains of lateral organs, and developing vascular tissues, while expression of ATHB8 is restricted to developing vascular tissues (Baima et al. 1995; McConnell et al. 2001; Emery et al. 2003; Prigge et al. 2005; Williams et al. 2005). During the development of leaf venation pattern, ATHB8-GUS is expressed at very early stages in positions where veins are predicted to appear, but before the diagnostic anatomical features of procambium are expressed (Kang and Dengler 2004; Scarpella et al. 2004). In developing leaves, linearly adjacent ground tissue cells initiate ATHB8 expression, and development is continuous and polar in the sense that the first cells to express ATHB8-GUS are adjacent to preexisting procambial strands and that ground cells at the terminus of a developing file are recruited to the ATHB8GUS-expressing file, extending it across a panel of ground tissue (Kang and Dengler 2004; Scarpella et al. 2004). This expression pattern within the ground meristem presages the anatomical emergence of procambium and has been termed ‘‘preprocambium’’ (Mattsson et al. 2003). Following the preprocambial stage of development, cells in the file acquire the distinctive anatomical features of procambium, and ATHB8-GUS expression increases (Kang and Dengler 2004; Scarpella et al. 2004). In contrast with the progressive appearance of the preprocambial phase, the emergence of procambium anatomy appears to occur simultaneously along the file of cells (Scarpella et al. 2004). As xylem and phloem cells gradually differentiate from procambial tissue, ATHB8-GUS expression becomes restricted to the residual procambium between the vascular tissues and undifferentiated cells on the adaxial (xylem) side of procambial strands; expression ceases in fully differentiated veins (Kang and Dengler 2002). Similarly, in stem vascular bundles, ATHB8 expression becomes restricted to a narrow zone of procambium between the differentiating xylem and phloem tissues (Baima et al. 1995). Thus, ATHB8 expression provides a uniquely suitable marker for analysis of vascular architecture, as it defines both an early prepattern and procambium itself throughout its development. Despite this distinctive expression pattern, the specific developmental function of ATHB8 is unknown (Emery et al. 2003; Prigge et al. 2005). Homozygous ATHB8 loss-offunction mutants have no detectable phenotype (Baima et al. 2001), while ectopic expression of ATHB8 results in proliferation of xylem precursor cells and subsequent increase in the numbers of mature xylem cells (Baima et al. 2001). In contrast, single loss- or gain-of-function mutations in other members of the class III HD-Zip gene family result in dramatic conversions of vegetative and floral lateral organ dorsiventral symmetry (McConnell et al. 2001; Emery et al. 2003; Prigge et al. 2005). Mutants of ATHB8 also have relatively little effect other than smaller stature in triple, quadruple, and quintuple combinations with other class III HD-Zip mutants (Prigge et al. 2005). There is some evidence, however, that ATHB8 may interact antagonistically with the REVOLUTA and ATHB15/CORONA loci as defects in the differentiation of sclerenchyma fibers from the ground tissue in inflorescence stems normally associated with these mutants are suppressed in triple mutants (Prigge et al. 2005). Members of the class III HD-Zip gene family # 2006 NRC Canada 1662 clearly have overlapping and redundant functions in meristem establishment, organ polarity, and vascular development, making it difficult to establish the functions of a single member such as ATHB8 through mutant analysis. Evidence of a function for ATHB8 in vascular patterning and development comes from observations of ATHB8 expression in response to the plant growth hormone auxin. ATHB8-GUS expression is upregulated in response to auxin treatment in wounded tobacco stems (Baima et al. 1995), and ATHB8 mRNA increases in Arabidopsis whole seedlings or detached leaves incubated with auxin (Baima et al. 1995; Mattsson et al. 2003). Additionally, ATHB8 transcript levels are reduced when the auxin response factor MONOPTEROS is impaired or are increased when MONOPTEROS is overexpressed (Mattsson et al. 2003). GUS expression driven by the synthetic auxin response element DR5 marks the preprocambial stage of leaf vein development (Mattsson et al. 2003), as does ATHB8-GUS (Kang and Dengler 2004; Scarpella et al. 2004). These observations indicate that ATHB8 expression might represent a downstream event in the process that translates an auxin signal into a procambial strand. The canalized flow of auxin through shoot tissues is hypothesized to be the specific signal that induces formation of a vascular strand (Sachs 1981, 1991). Support for this model comes from experiments demonstrating that an artificial auxin source can induce the formation of a vascular strand within wounded stem tissue (reviewed in Sachs 1981; Lyndon 1990). Numerous physiological experiments have shown that auxin moves basipetally within intact stems (reviewed in Lomax et al. 1995) and that movement is dependent on the cellular localization of a plasma membranebound protein ARABIDOPSIS THALIANA PIN-FORMED 1 (ATPIN1) (reviewed in Paponov et al. 2005). ATPIN1 is one of eight PIN genes present in the Arabidopsis genome (Paponov et al. 2005). The encoded PIN proteins have been shown to be required for normal plant embryogenesis, organogenesis, phototropism, and gravitropism and are thought to act through a role in polar auxin transport, although it is currently unknown whether they act as auxin efflux carriers directly or as regulators of polar auxin transport (Paponov et al. 2005). Nevertheless, polar auxin movement is dependent on the polar localization of ATPIN1 protein within the cell (Galweiler et al. 1998; Benková et al. 2003). The developmental responses to polar auxin movement and signaling are not simple, as a complex series of coordinated cell divisions, cell enlargement, and patterned differentiation events are required to produce the highly organized and functional vascular strand rather than a broad unpatterned zone of vascular cells (Berleth and Mattsson 2000; Berleth et al. 2000). A role for ATHB8 in this process is still a putative one, but one that we have exploited for a characterization of primary vascular architecture in the compressed, miniature shoot of Arabidopsis. Phyllotaxis and shoot vascular architecture The pattern of initiation of leaf primordia on the flanks of the SAM gives rise to one of the most conspicuous features of whole-shoot morphology, phyllotaxis. Phyllotactic patterns generally are regular (although exceptions occur: Kelly and Cooke 2003; Jeune and Barabé 2004), and individual Can. J. Bot. Vol. 84, 2006 species or whole taxonomic groups are characterized by specific patterns, usually helical, distichous, decussate, or whorled. Developmental shifts in phyllotaxis may occur, such as those associated with shoot phase change from juvenile to adult or from vegetative to reproductive (Poethig 1990; Kwiatkowska 1995). Helical phyllotaxis is the most common pattern among dicotyledons and has received the most attention in terms of analysis of the geometrical patterns and modeling pattern generation within the SAM (Richards 1951; Mitchison 1977; Steeves and Sussex 1989; Lyndon 1990, 1998; Jean 1994; Adler et al. 1997; Reinhardt and Kuhlemeier 2002; Smith et al. 2006; Jönsson et al. 2006). In species with helical phyllotaxis, leaf primordia are initiated at a more or less constant divergence angle (approximately 137.58) along a shallow helix, the ontogenetic (or ‘‘genetic’’) helix. Additional helices that are steeper than the ontogenetic helix, the parastichies, can be recognized on the exterior of the shoot and, more readily, in transverse sections of the shoot apex region (Esau 1965a, 1965b; Beck et al. 1982; Kirchoff 1984) (Fig. 1A). Leaf primordia that are in direct contact form conspicuous contact parastichies, while steeper noncontact parastichies can also be recognized (Kirchoff 1984). The serial positions of leaves within a parastichy as well as the numbers of clockwise and anticlockwise parastichies are integers belonging to the Fibonacci summation series (Richards 1951; Mitchison 1977; Esau 1965a, 1965b). Knowledge of specific parameters of a helical phyllotactic system makes it possible to predict the position of the next leaf primordium to be initiated on the flanks of the SAM with considerable accuracy. Similarly, knowledge of phyllotaxis permits predictions about the placement of vascular bundles within the stem (Girolami 1953; Skipworth 1962; Philipson and Balfour 1963; Esau 1965a, 1965b; Beck et al. 1982; Kirchoff 1984). The longitudinal vascular bundles of the stem (here referred to as vascular sympodia and most clearly recognizable in immature portions of the stem) branch at intervals to give rise to the leaf traces, the individual smaller vascular bundles that supply the leaves. The number of vascular sympodia usually reflects phyllotaxis: for instance, plants with distichous or decussate phyllotaxis typically have even numbers of vascular sympodia (e.g., 4, 6), while plants with helical phyllotaxis have a number of sympodia belonging to the Fibonacci summation series (e.g., 5, 8, 13: Beck et al. 1982; Kirchoff 1984). In species with a single trace supplying each leaf (the common condition: Esau 1965a, 1965b; Beck et al. 1982), leaves belonging to one parastichy all derive their traces from the same vascular sympodium. In species with three or more traces per leaf, the central traces are supplied from the same vascular sympodium, while the lateral traces are derived from adjacent sympodia (e.g., Larson 1975). In some species, there are no interconnections between adjacent vascular sympodia (an open pattern), so that each vascular sympodium extends in a steep helix that mirrors one parastichy on the exterior of the stem and branches to give rise to leaf traces at regular intervals. This simple, open pattern gives rise to the sectoriality observed in some studies of long-distance transport of water, solutes, and signaling molecules (Marshall 1996; Orians and Jones 2001). In other species, regular anastomoses between vascular sympodia form a closed, reticulate pattern in which leaf traces are derived as # 2006 NRC Canada Dengler 1663 Fig. 1. Phyllotaxis and primary vascular architecture in a vegetative shoot of Arabidopsis. (A) Cross section at the level of the shoot apical meristem. Rosette leaves are numbered according to order of ontogenetic helix. The n + 3 and n + 5 contact parastichies are indicated, as are the steeper n + 8 and n + 13 noncontact parastichies. (B) Cross section at 66 mm below the shoot apical meristem. Vascular bundles represent either individual leaf traces (3, 4, 5, 6, 7) or vascular sympodia that will give rise to multiple leaf traces (8, 9, 10, 11, 12). Solid lines, clockwise parastichies; broken lines, anticlockwise parastichies. Scale bar = 50 mm. (From Kang et al. 2003, reproduced with permission of the New Phytol. 158: 53–64). branches of two adjacent vascular sympodia. Interestingly, a correlation between the relative timing of the onset of cambial activity and the nature of the vascular system has been noted in a modest species sample: secondary vascular development is initiated early when primary vascular architecture is open, but initiated late and (or) is limited in extent when primary vascular architecture is closed (Dormer 1945, 1946; Philipson and Balfour 1963). Primary vascular architecture in Arabidopsis: mature pattern Vegetative rosette Based on analysis of the patterns of ATHB8-GUS expression and of anatomically defined procambium and (or) vascular tissues, vascular architecture of the vegetative rosette of Arabidopsis is a closed, reticulate pattern that corresponds closely to phyllotaxis (Figs. 1 and 2) (see Kang et al. 2003). In vegetatively growing shoots with 20 or more leaves, the most conspicuous contact parastichies are those connecting every third leaf (n + 3) and every fifth leaf (n + 5) of the ontogenetic helix (Fig. 1A). These contact parastichies extend up the shoot axis in either a clockwise or a counterclockwise direction (n + 5 and n + 3, respectively, in Fig. 1A), depending on the overall orientation of shoot helical phyllotaxis (anticlockwise for the shoot illustrated in Fig. 1A). Individual rosettes with clockwise or anticlockwise helical phyllotaxis occur with approximately equal frequencies in Arabidopsis (Kang et al. 2003). Generally, three n + 3 contact parastichies (connecting leaves 7, 10, 13, 16, etc., leaves 5, 8, 11, 14, etc., leaves 6, 9, 12, 15, etc.) and five n + 5 contact parastichies (connecting leaves 5, 10, 15, etc., leaves 6, 11, 16, etc., leaves 7, 12, 17, etc., leaves 8, 13, 18, etc., leaves 9, 14, 19, etc.) are present. In addition to these more obvious contact parastichies, steeper, noncontact parastichies (n + 8, n + 13; Fig. 1A) can be superimposed on overall shoot helical phyllotaxis. Geometrical properties such as these intersecting parastichies and other properties of leaf arrangement accurately predict the spatial positioning of vascular bundles within the stem. The vascular bundles seen in any one cross section of the stem represent either individual leaf traces (4 in Fig. 1B) or the vascular sympodia that branch and give rise to the individual leaf traces (e.g., branches from the sympodium labeled 9 in Fig. 1B give rise to the traces of leaves 9, 14, and 17). The levels of branching of parent sympodia and the divergence of leaf traces determine the number of vascular strands observed in individual transverse sections, but the number in wild-type Arabidopsis is typically eight (Fig. 1B). When individual vacular bundles are traced through successive serial sections, they can be seen to branch and give rise to the traces of leaves that are positionally related in the n + 8 and n + 5 parastichies. For instance, the sympodium supplying leaf 12 is derived from branches of bundles supplying leaf 4 (antecedent in n + 8 parastichy) and leaf 7 (antecedent in n + 5 parastichy) (Fig. 2), forming an anastomosing pattern and the closed pattern of primary vascular architecture that characterizes Arabidopsis vegetative growth (Fig. 2). Thus, vascular architecture of the Arabidopsis shoot is a # 2006 NRC Canada 1664 Can. J. Bot. Vol. 84, 2006 Fig. 2. Idealized two-dimensional diagram representing primary vascular architecture in vegetative shoots of Arabidopsis. Foliage leaves are represented by squares, numbered according to ontogenetic helix. Squares represent the approximate level of the leaf base. Leaf traces are derived as branches of vascular sympodia supplying antecedent leaves in the n + 8 (blue) and n + 5 (green) parastichies, a pattern established only after the second leaf in each n + 8 parastichy. Leaf traces are detectable during P1 (leaf 19) as branches from the antecedent leaf trace/sympodium in the n + 8 parastichy, while the n + 5 connections are present 3–4 plastochrons later (leaf 16). Branches connecting sympodia are shown as horizontal, reflecting the vertically compressed architecture of the rosette. Vascular bundles connected to antecedent sympodia are illustrated as double cylinders, although they are anatomical coherent (see Fig. 1B); those connected to one antecedent sympodium are illustrated as one. The blue line at the base of the illustration represents the solid cylinder of the hypocotyl vasculature, which gives rise directly to cotyledonary and juvenile leaf traces (see Busse and Evert 1999). Not to scale. highly integrated system in which the vascular supply to each leaf is directly connected to antecedent leaves in two different parastichies, providing alternative pathways for the long-distance movement of water and solutes. Although the fully expressed primary vascular architecture is a closed, reticulate system, the formation of the anastomosing procambial strands is not synchronous in that the vascular connection with the n + 5 antecedent leaf lags behind the n + 8 connection during development. When a leaf trace is first detectable, it extends as a branch of the trace of its n + 8 antecedent leaf and terminates just below its corresponding primordium within the SAM (see Kang et al. 2003). Connections with the n + 5 antecedent leaf trace are formed three to four plastochrons later; thus, on a developmental basis, shoot primary vascular architecture is initially an open system but quickly becomes modified as a closed reticulum (summarized in Fig. 2). The closed, reticulate pattern of vascular architecture with regular connections between the sympodia corresponding to the n + 5 and n + 8 parastichies characterizes the adult phase of shoot ontogeny. During the juvenile phase, however, phyllotaxis is subdecussate, and the traces to leaves 1 to 4 arise directly from the vascular cylinder of the hypocotyls, as do those of the cotyledons (see Busse and Evert 1999; Kang et al. 2003) (Fig. 2). Branches from these traces anastomose to form the traces of the first-formed adult leaves, and connections are initially between the sympodia corresponding to the n + 3 and n + 5 parastichies. The for- mation of leaf 9, which establishes the first n + 8 parastichy, initiates the fully expressed pattern summarized in Fig. 2. Inflorescence Upon the induction of reproductive growth, the SAM initiates floral meristems in place of leaves along the ontogenetic helix of the vegetative rosette (Fig. 3). In Arabidopsis plants grown under long-day inductive conditions, floral meristems are first formed at position 10, 11, or 12 (12 in Fig. 3A and 10 in Fig. 4; see Kang et al. 2003). At first, the vascular architecture of the inflorescence extends the reticulate pattern of the vegetative rosette, with floral meristem traces derived from those of the antecedent organs in the n + 8 and n + 5 parastichies. For instance, in the inflorescence illustrated in Fig. 4, the sympodium supplying flower 12 is derived from those supplying leaves 4 (n + 8 parastichy) and 7 (n + 5 parastichy). After formation of the second flower in each n + 5 parastichy, however, vascular architecture switches to an open pattern, with primary connections along the n + 5 parastichies (e.g., flower 17, Fig. 4). Although the basic pattern follows that of the vegetative rosette, the inflorescence pattern differs in two specific ways: (i) the flower trace connects with the trace/ sympodium corresponding to the n + 5, not the n + 8 parastichy, and (ii) a second connection with the adjacent sympodium was not detected, resulting in a primarily open pattern of inflorescence vascular architecture (summarized in Fig. 4). ATHB8-GUS expression within new procambial # 2006 NRC Canada Dengler 1665 Fig. 3. Phyllotaxis and primary vascular architecture in the inflorescence of Arabidopsis. (A) Cross section at the level of the shoot apical meristem. Rosette leaves (5–8), cauline leaves (9–11), and floral meristems (12–21) are numbered in order of formation on the ontogenetic helix. The n + 3 and n + 5 contact parastichies are indicated, as are the steeper n + 8 and n + 13 noncontact parastichies. (B) Cross section at 490 mm below the shoot apical meristem. Rosette leaves 5–8 and the trace/sympodia supplying cauline leaves 9–11 and flowers 12–15 are numbered. Note the axillary buds associated with rosette leaves 5–8 and axillary bud leaf primordia (arrows). Scale bar = 50 mm. (From Kang et al. 2003, reproduced with permission of the New Phytol. 158: 53–64). strands is weaker in the inflorescence apex than in vegetative apices, but the timing in relation to primordium formation and the longitudinal pattern of ATHB8-GUS expression appears to be similar (Kang et al. 2003). Although inflorescence vascular architecture is initially an open system, secondary modifications reinstate the closed reticulate nature of the shoot’s primary vascular architecture. Accessory traces form bridges between individual flower bud traces and the adjacent n + 3 and n + 5 sympodia (Fig. 4). Such accessory traces are formed relatively late (usually 10 or more plastochrons after floral meristem initiation), are narrow in diameter relative to the floral meristem traces, and have a horizontal course. Axillary buds Upon transition from the vegetative to the reproductive phase, axillary buds are initiated basipetally, starting with the cauline leaves (Long and Barton 2000). Axillary bud meristems initially lack detectable vascular bundles, but the appearance of two short procambial strands is coincident with formation of the first two leaf primordia on the axillary shoot (see Kang et al. 2003). These procambial strands are not isolated but appear to be continuous as branches of either the leaf trace alone (most rosette leaves) or the adjacent vascular sympodia (cauline leaves) or a combination of the two patterns (summarized in Fig. 4; Kang et al. 2003). Thus, the vascular connections for the primary inflorescence branches associated with the cauline leaves are well integrated with at least three vascular sympodia, providing a built-in redundancy of vascular pathways supplying the flowers and developing siliques born on those branches if individual vascular bundles are damaged. The stepwise elaboration of the primary vascular system in vegetative and reproductive shoots of Arabidopsis involves the SAM itself as well as older regions of the stem. Formation of the leaf trace (derived from the n + 8 sympodium) occurs during the first plastochron (P1), while the connection with the n + 5 sympodium occurs later (P3 or P4). Vascular connections between axillary buds and adjacent vascular sympodia develop outside the SAM in older tissues (depending on growth conditions), as do those that connect the traces of developing flower buds with adjacent sympodia. These later developmental events are superimposed on the initial primary pattern and presumably require comparable developmental signals and signaling pathways, although the developmental environment in terms of overall tissue differentiation differs from that within the SAM. The progressive elaboration of the primary vascular system allows plants to respond appropriately to variation in the growth environment in that the pattern may be arrested at a simple phase when plants are short-lived or elaborated when growth is prolonged. Under some growth conditions, secondary vascular development occurs within the rosette and the basal portion of the inflorescence axis (Altamura et al. 2001; Chaffey et al. 2002), replacing the primary vascular system functionally. The basic features of vascular development described for the rosette plant Arabidopsis on the basis of ATHB8-GUS expression correspond to descriptions of elongate shoots in other species studied, most notably for Linum usitatissimum L. (Linaceae) (Girolami 1953), Hectorella caespitosa J.D. Hooker (Portulaceae) (Skipworth 1962), and Populus deltoides Bartr. ex Marsh. (Saliceae) (Larson 1975). In herbaceous Linum and Hectorella, primary vascular architecture is a closed, reticulate system with single acropetally developing leaf traces connected to vascular sympodia that correspond to parastichies. As these shoots mature, the numbers of parastichies (and the numbers of intervening leaves along a parastichy: n + 13, n + 21, etc.) and vascular sympodia increase, just as seen for the juvenile to adult transitions in Arabidopsis. In woody Populus, vascular architecture is a more complex, open system with three traces per leaf, yet procambial strands develop acropetally and precisely according to phyllotaxis (Larson 1975, 1977). In Populus, vas# 2006 NRC Canada 1666 Can. J. Bot. Vol. 84, 2006 Fig. 4. Idealized two-dimensional diagram representing primary vascular architecture in the Arabidopsis inflorescence. Foliage leaves are represented by squares and flowers by hexagons, numbered according to the ontogenetic helix; symbols represent the approximate level of the primordium base. Axillary buds (triangles) are associated with cauline leaves 7–9 and with rosette leaves 3–6. After the second flower in each n + 5 parastichy, traces are derived as branches of vascular sympodia supplying antecedent flowers in the n + 5 parastichy, forming an open pattern. Accessory bundles (yellow) connect flower traces with adjacent sympodia after seven or more plastochrons. Axillary bud procambial strands (purple) form as branches of either the leaf trace (leaves 3–6) or adjacent sympodia (9) or a combination of the two (7, 8). The blue line at the base of the illustration represents the solid cylinder of the hypocotyl vasculature. Not to scale. culature may be elaborated secondarily by the formation of accessory bundles that provide additional connections between leaf traces and adjacent vascular sympodia (Larson 1980a, 1980b). Development of vascular architecture and expression of ATHB8 Despite the progressive elaboration of primary vascular architecture within rosette and inflorescence stems of Arabidopsis, the developmental steps taking place within the SAM itself are similar during both vegetative and reproductive phases. Both leaf primordia and floral meristems are supplied by a single trace that appears to develop acropetally and in continuity with pre-existing vasculature and is positioned precisely in relation to earlier-formed vascular bundles and to the placement of newly formed lateral organ primordia. Based on ATHB8 expression, procambial strand (or at least preprocambial strand) formation appears to be coincident with the initial formation of an externally detectable primordium (P1), although detection of primordia from serial cross sections may have identified only slightly older primordia (P2 or P3; Kang et al. 2003). During the vegetative stage of development, procambial strands are continuous (based on a combination of anatomical criteria and ATHB8 expression), with the trace/sympodium procambial strand corresponding to the n + 8 parastichy. Although this pattern shifts subtly in the inflorescence (connections correspond to the n + 5 parastichy), the formation of new strands follows a highly predictable pattern with no indication of a stochastic component to linkages between strands. The con- tinuous, acropetal nature of procambial strand formation appears to be general for the dicotyledons, virtually without exception (Esau 1965a, 1965b) and has been previously reported for Arabidopsis (Vaughan 1955; Busse and Evert 1999). Although procambial strands within the vegetative and reproductive shoot apical meristems of Arabidopsis appear to be continuous with antecedent procambial strands, the zone of ATHB8-GUS expression is initially discontinuous (Kang et al. 2003). The longitudinally oriented narrow files of cells below each new primordium express the ATHB8-GUS construct strongly, while ATHB8-GUS activity initially is not detectable at the basal end where these strands curve to connect with the antecedent parent strand. This stage is ephemeral, as ATHB8-GUS expression becomes established throughout the length of the leaf trace as the procambial strand grows in diameter. Such a pattern could indicate that ATHB8 expression is induced by a basipetally moving signal and that the discontinuity reflects a moving front that interacts with acropetally moving signals, possibly traveling in the phloem (reviewed in Lough and Lucas 2006). This idea is highly speculative, however, as the function of ATHB8 is still not established; for instance, ATHB8 expression might simply be a marker of acquisition of xylem cell identity and only presages the discontinuous nature of xylem differentiation (Kang et al. 2003). An auxin model that integrates phyllotaxis and vascular development The SAM generates regular, predictable patterns of leaves # 2006 NRC Canada Dengler and other lateral organs that are generally robust to experimental manipulation (e.g., Reinhardt et al. 2003, 2005; Smith et al. 2006). Phyllotaxis and the three-dimensional architecture of the vasculature supplying the lateral organs are highly coordinated in their development. Although the nature of this coordinated development is not yet fully understood, current models point to mechanisms that regulate the positioning of leaf and floral primordia and of their vascular supply in an integrated manner. Earlier models of the regulation of phyllotaxis fall into two broad categories: in some (i), interactions among primordia on the flanks of the shoot apical meristem are thought to determine the placement of leaves and flowers, while in others (ii), inductive signals from older portions of the shoot are thought to play a role (Larson 1983; Jean 1994; Lyndon 1998; Reinhardt and Kuhlemeier 2002; Reinhardt et al. 2003). The nature of interactions among primordia is thought to be either biophysical (e.g., Green 1996) or biochemical (e.g., Mitchison 1977; Schwabe 1984) and to display features of a lateral inhibition system in which new primordia are placed at the maximum distance possible from the ‘‘inhibitory’’ antecedent primordia on the flanks of the meristem (Lyndon 1990; Meinhardt 1996). Observations that the placement of new primordia merely reiterates that of older portions of the shoot and that developing vascular strands sometimes precede the external appearance of the primordia that they will supply have led to suggestions that inductive signals move acropetally through the vascular system (discussed in Esau 1965a, 1965b; Larson 1983; Lyndon 1990). Current evidence, however, strongly supports a model in which the phyllotactic pattern is generated within the SAM through biochemical interactions of pre-existing primordia, specifically through regulation of local variation in auxin concentration. The auxin model postulates that auxin concentrations act, not as inhibitors of primordium development, but as active effectors of a specific developmental sequence of events (Reinhardt et al. 2003; Reinhardt 2005; Fleming 2004, 2005; Heisler et al. 2005; Smith et al. 2006; Jönsson et al. 2006). The key features of this model (summarized in Fig. 5) are (i) uniform acropetal movement of auxin in the surface (L1) layer of the apical meristem from regions outside the SAM, (ii) formation of foci of auxin concentration in positions that presage the position of primordia through the polar localization of ATPIN1 proteins, and (iii) redistribution of ATPIN1 polarity upon the outgrowth of primordia so that polar auxin movement is directed toward the interior of the meristem, along a narrow file of cells in the position of the future midvein procambial strand. Support for this model comes from a number of sources. First, when polar auxin transport is inhibited by pharmacological treatments, primordium formation is suppressed (Reinhardt et al. 2000; Stieger et al. 2002; Benková et al. 2003). Second, as shown for inhibitor-treated tomato shoot tips or pin1 mutants of Arabidopsis, suppression of primordium formation can be reversed by localized auxin treatment (Reinhardt et al. 2000). Third, the auxin efflux carrier protein ATPIN1 accumulates in the L1 layer of the meristem, specifically on the acropetal side of individual cells, indicating that net movement of auxin is toward the center of the SAM (Benková et al. 2003; Reinhardt et al. 2003; Heisler et al. 2005). Fourth, ATPIN1 protein accumulation is strongest in posi- 1667 tions that anticipate primordium formation by one to three plastochrons (Reinhardt et al. 2003), as is AUXIN RESISTANT 1, a putative auxin influx carrier (Stieger et al. 2002; Benková et al. 2003). Expression of the reporter construct DR5-GFP, thought to reflect the endogenous auxin concentration (Benková et al. 2003), also is restricted to the L1 layer of the SAM and expression peaks in incipient primordia (Smith et al. 2006). The phyllotactic pattern of ATPIN1 accumulation is disrupted in the mutants pin1, pinoid, and monopteros, indicating that auxin signaling and transport are required for correct PIN1 localization (Reinhardt et al. 2003). Fifth, ATPIN1 protein is dynamically redistributed during each plastochron: ATPIN1 polarity is first directed toward the center of the presumptive primordium (I1 in Fig. 5), but with outgrowth, L1 layer expression decreases and a narrow file of internal cells begins to accumulate ATPIN1 with a basipetal polarity. (Reinhardt et al. 2003; Heisler et al. 2005; P1 in Fig. 5). Live imaging of ATPIN1 distribution within the Arabidopsis floral meristem also shows that ATPIN1 concentration is highest in the I3, I2, and I1 presumptive floral meristem sites, when the polarity of surface cells adjacent to older, antecedent primordia is strongly directed toward the center of the presumptive primordium (Heisler et al. 2005). With the outgrowth of the primordium, ATPIN1 becomes localized to the basal ends of the internal cells forming a narrow file (Heisler et al. 2005). Thus, the positive induction of primordium position and vascular strand position by the localized regulation of auxin concentrations could provide a single mechanism that regulates both phyllotaxis and vascular architecture, much as hypothesized by Esau (1965a). The auxin model provides a mechanism for the formation of leaf trace procambial strands in a pattern that is coordinated with phyllotaxis, but almost all aspects of how the initial pattern of ATPIN1 distribution is translated into the complex three-dimensional vascular architecture of the shoot are unknown. In many ways, the localization of ATPIN1 protein to an internal strand of cells during the early plastochrons of primordium development (Reinhardt et al. 2003; Heisler et al. 2005) is comparable with the generation of the two-dimensional, yet complex, pattern of leaf veins. In developing leaves, PIN1 is expressed first in the protoderm (derived from the L1 layer) and PIN1-GFP protein is localized subcellularly to the acropetal side of protodermal cells, producing a convergence point at the apex (Scarpella et al. 2006); thus, the pattern observed for the SAM is preserved during early stages of leaf development. Internal cells adjacent to the apical convergence point accumulate PIN1-GFP at the basal side of the cells, and the zone of expressing cells extends towards the base of the leaf, forming the midvein prepattern (Scarpella et al. 2006). The looped secondary veins are generated similarly, starting with a convergence point at the leaf margin and with a series of cells expressing PIN1-GFP extending toward the midvein. These cells accumulate PIN1-GFP on the side toward the midvein, suggesting that auxin moves along the incipient vein from a source at the margin to the sink represented by the earlier-formed strand (Scarpella et al. 2006). Strands of preprocambial tissue are polar in their development and extend unidirectionally from pre-existing strands; if strand extension is arrested, a ‘‘freely ending veinlet’’ is # 2006 NRC Canada 1668 Can. J. Bot. Vol. 84, 2006 Fig. 5. Idealized two-dimensional diagram representing the spatial pattern of polar auxin flow (based on localization of the ATPIN1 protein: Reinhardt et al. 2003; Heisler et al. 2005) and of ATHB8 expression (based on promoter-GUS expression: Kang et al. 2003) in vegetative and inflorescence SAMs of Arabidopsis. Arrows, coinciding with localization of ATPIN1, indicate net movement of auxin toward the centers of incipient primordia (I1, I2) and toward internal tissue during early primordium outgrowth (P1, P2). An isolated file of ATHB8-GUSexpressing cells (blue) is present at the P1 and later stages below the base of the primordium, although a connecting strand of anatomically defined procambium (pink) is consistently present, connecting it to the antecedent procambial strand associated with a leaf that is eight plastochrons older (vegetative SAM). formed. During the development of most of the secondary and higher-order venation, however, strand extension rapidly connects with an adjacent strand, thus forming a continuous link between pre-existing strands (Scarpella et al. 2004, 2006). The prepatterns represented by PIN1-GFP, the auxin reporter DR5-GUS, the gene trap marker ET1335-GUS, or ATHB8-GUS all appear when the leaf (or field of tissue for higher-order veins) consists of relatively few cells and is extended by intercalary growth as the leaf enlarges (Mattsson et al. 2003; Kang and Dengler 2004; Scarpella et al. 2004, 2006); thus, the stage of discontinuity and unidirectional growth is ephemeral (except for the freely ending veinlets). The cytological features of cells expressing these markers are indistinguishable from those of cells of the ground tissue in which this preprocambial pattern arises; only later do the distinctive elongate shape and dense cytoplasm of anatomically defined procambial cells emerge (Mattsson et al. 2003; Kang and Dengler 2004; Scarpella et al. 2004, 2006). Thus, elements of pattern formation are shared by development of both two-dimensional leaf vein patterns and threedimensional shoot vascular pattern: (i) unidirectional elaboration of the system that may reflect auxin transport from localized sources to sinks within the tissue, (ii) formation of major elements of the system, such as leaf primary and secondary veins or the n + 8 sympodial strands of the stem followed by formation of minor pattern elements, such as the higher order venation or the n + 5 sympodial strands, and (iii) discontinuities in the accumulation pattern of certain proteins, such as that observed for PIN1 expression in an in- cipient leaf primordium (Reinhardt et al. 2003; Heisler et al. 2005) or in the expression of ATHB8 within leaf traces (Kang et al. 2003). An important distinction between the two is that while leaf vein pattern development points to a highly flexible self-organizing patterning mechanism (Scarpella et al. 2006), shoot vascular architecture appears to be highly predictable, with almost no stochastic element to the formation of connections between strands (Kang et al. 2003). Future prospects Plants are increasingly regarded as supracellular organisms in which long-distance transport of, not only water and dissolved nutrients, but also of macromolecules and other signaling substances depends on the vascular system (Lough and Lucas 2006). Knowledge of the three-dimensional architecture of the vascular system, and particularly how it corresponds to leaf position on the shoot, is essential for understanding how photosynthate moves through the phloem from specific source to specific sink leaves or how chemical defense signals might move from herbivore-damaged leaves to particular newly expanding ones (e.g., Larson 1977; Marshall 1996; Orians and Jones 2001). For instance, in poplar, when 14C label was supplied to a photosynthesizing source leaf, it was possible to predict where the relative percentages of the labeled photosynthates would appear based on knowledge of the connections of central and lateral leaf traces with adjacent vascular sympodia and of the transitions in phyllotaxis appearing during shoot ontogeny # 2006 NRC Canada Dengler (Larson 1977). Analyses of phloem sap and use of grafting between stocks and scions of different genotype have demonstrated that numerous macromolecules function in longdistance communication and use phloem tissue as a conduit (reviewed by Lough and Lucas 2006). One example of these is the FLOWERING LOCUS T protein, a component of a phloem-mobile florigenic signal. Induction of gene expression in a source leaf by heat shock results in accumulation of FLOWERING LOCUS T mRNA in both the source leaf and the SAM, indicating that the mRNA (or perhaps the protein) moves along the vascular sympodium(a) connecting the source leaf and the primordia closest to the SAM (Huang et al. 2005). Although many environmental and developmental stimuli may not be localized, so that signaling moves through the entire shoot vasculature and affects leaves of all parastichies equally, many triggers will be specific to regions of the shoot or root system, and vascular architecture will contribute to determining which targets will be reached by those signals. Knowledge of shoot primary vascular architecture of Arabidopsis, specifically, might form the basis for better understanding aspects of the developmental biology of this model organism. The information described herein is especially useful for the interpretation of mutations that affect the vascular system, for instance the supernumerary vascular bundles that appear in mutants such as REVOLUTA (Zhong et al. 1999) or the effects of mutants in other HD-ZIP family genes on shoot vascular pattern as well as ground sclerenchyma (Prigge et al. 2005). Expression patterns of genes such as ATHB8 or DR5 that are restricted to developing procambial strands might be used in mutant screens to identify genes that function in the construction of this complex three-dimensional pattern. Although the analysis presented here was based on laborious reconstruction from serial cross sections (Kang et al. 2003), analysis of cleared whole shoots of plants carrying these reporter constructs or use of confocal microscopy and other imaging systems would allow identification of mutants. Most importantly, knowledge of the primary vascular system of Arabidopsis and how it remains coordinated with changes in phyllotaxis and organ identity through juvenile, vegetative adult, and reproductive phases will contribute to understanding the genetic framework that underlies shoot development. In summary, developmental biologists have made dramatic headway in providing evidence from gene reporter studies, experimental manipulations, and modeling that strongly support the role of the PIN proteins in the regulation of localized auxin concentrations, leading to the patterned initiation of leaf primordia in the correct phyllotactic sequence on the flanks of the SAM. Tantalizingly for the topic at hand, the subcellular localization of PIN1 proteins in Arabidopsis indicates that auxin flows from the center of the primordium site inwards along a narrow path, presaging the location of the leaf midvein and its connecting leaf trace. Thus far, imaging techniques have been limited to surface layers, so it has not been possible to ‘‘connect the dots’’ and relate this midvein prepattern with the predicted connection of the leaf trace with the vascular sympodium corresponding to the n + 8 parastichy. Detailed analysis of the development of leaf vein pattern indicates that localized concentrations of auxin also initiate the expression of a pro- 1669 cambial prepattern that connects a source of auxin with tissues acting as a sink and that reiterations of the process create a complex hierarchical pattern (Scarpella et al. 2006). Although such concentrations only have been shown to occur in the L1 layer of the SAM and the protoderm of developing leaves, a similar process is likely to occur within internal ground tissues and would be integral to the generation of the three-dimensional vascular pattern of shoots and to its secondary modification during development. Acknowledgments I thank Thomas Berleth, Julie Kang, Bill Remphrey, and two anonymous reviewers for helpful comments on the manuscript, Janice Wong for illustrations, and the Natural Sciences and Engineering Research Council of Canada for research support. References Adler, I., Barabé, D., and Jean, R.V. 1997. A history of the study of phyllotaxis. Ann. Bot. (Lond.), 80: 231–244. Altamura, M.M., Possenti, M., Matteuci, A., Baima, S., Ruberti, I., and Morelli, G. 2001. Development of the vascular system in the inflorescence stem of Arabidopsis. New Phytol. 151: 381–389. doi:10.1046/j.0028-646x.2001.00188.x. Baima, S., Nobili, F., Sessa, G., Lucchette, S., Ruberti, I., and Morelli, G. 1995. The expression of the Athb-8 homeobox gene is restricted to provascular cells in Arabidopsis thaliana. Development, 121: 4171–4182. PMID:8575317. Baima, S., Possenti, M., Matteucci, A., Wisman, E., Altamura, M.M., Ruberti, I., and Morelli, G. 2001. The Arabidopsis ATHB-8 HD-Zip protein acts as a differentiation-promoting transcription factor of the vascular meristem. Plant Physiol. 126: 643–655. doi:10.1104/pp.126.2.643. PMID:11402194. Baurle, I., and Laux, T. 2003. Apical meristems: the plant’s fountain of youth. Bioessays, 25: 961–970. doi:10.1002/bies.10341. PMID:14505363. Beck, C.B., Schmid, R., and Rothwell, G.W. 1982. Stelar morphology and the primary vascular system of seed plants. Bot. Rev. 48: 691–816. Benková, E., Michniewicz, M., Sauer, M., Teichmann, T., Seifertová, D., Jűrgens, G., and Friml, J. 2003. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell, 115: 591–602. doi:10.1016/S0092-8674(03)00924-3. PMID:14651850. Berleth, T., and Mattsson, J. 2000. Vascular development: tracing signals along veins. Curr. Opin. Plant Biol. 3: 406–411. doi:10. 1016/S1369-5266(00)00104-7. PMID:11019809. Berleth, T., Mattsson, J., and Hardtke, C.S. 2000. Vascular continuity and auxin signals. Trends Plant Sci. 5: 387–393. doi:10.1016/ S1360-1385(00)01725-8. PMID:10973094. Busse, J.S., and Evert, R.F. 1999. Vascular differentiation and transition in the seedling of Arabidopsis thaliana (Brassicaceae). Int. J. Plant Sci. 160: 241–251. doi:10.1086/314117. Carles, C.C., and Fletcher, J.C. 2003. Shoot apical meristem maintenance: the art of a dynamic balance. Trends Plant Sci. 8: 394–401. doi:10.1016/S1360-1385(03)00164-X. PMID:12927973. Chaffey, N., Cholewa, E., Regan, S., and Sundberg, B. 2002. Secondary xylem development in Arabidopsis: a model for wood formation. Physiol. Plant. 114: 564–600. DeBary, A. 1884. Comparative anatomy of the vegetative organs of phanerogams and ferns [English translation]. Oxford University Press, Oxford, UK. # 2006 NRC Canada 1670 Dormer, K. 1945. An investigation of the taxonomic value of shoot structure in angiosperms with special reference to Leguminosae. Ann. Bot. (Lond.), 9: 141–153. Dormer, K. 1946. Anatomy of the primary vascular system in dicotyledonous plants. Nature, 58: 737–739. Emery, J.F., Floyd, S.K., Alvarez, J., Eshed, Y., Hawker, N.P., Izhaki, A., Baum, S.F., and Bowman, J.L. 2003. Radial patterning of Arabidopsis shoots by Class III HD- ZIP and KANADI genes. Curr. Biol. 13: 1768–1774. doi:10.1016/j.cub.2003.09. 035. PMID:14561401. Esau, K. 1965a. Plant anatomy. John Wiley & Sons, New York. Esau, K. 1965b. Vascular differentiation in plants. Holt, Rinehart and Winston, New York. Fleming, A.J. 2004. The control of leaf development. New Phytol. 166: 9–20. Fleming, A.J. 2005. Formation of primordia and phyllotaxy. Curr. Opin. Plant Biol. 8: 53–58. doi:10.1016/j.pbi.2004.11.013. PMID:15653400. Floyd, S.K., and Bowman, J.L. 2004. Ancient microRNA targets sequences in plants. Nature, 428: 485–486. doi:10.1038/ 428485a. PMID:15057819. Galweiler, L., Guan, C., Műller, A., Wisman, E., Mendgen, K., Yephremov, A., and Palme, K. 1998. Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science, 282: 2226–2230. PMID:9856939. Girolami, G. 1953. Relation between phyllotaxis and primary vascular organization in Linum. Am. J. Bot. 40: 618–625. doi:10. 2307/2438450. Green, P.B. 1996. Expression of form and pattern in plants — a role for biophysical fields. Semin. Cell Dev. Biol. 7: 903–911. doi:10.1006/scdb.1996.0110. Heisler, M.G., Ohno, C., Das, P., Sieber, P., Reddy, G.V., Long, J.A., and Meyerowitz, E.M. 2005. Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem. Curr. Biol. 15: 1899–1911. doi:10.1016/j.cub.2005.09.052. PMID:16271866. Huang, T., Böhlenius, H., Eriksson, S., Rarcy, F., and Nilsson, O. 2005. The mRNA of the Arabidopsis gene FT moves from leaf to shoot apex and induces flowering. Science, 309: 1694–1696. doi:10.1126/science.1117768. PMID:16099949. Jean, R.V. 1994. Phyllotaxis: a systematic study in plant morphogenesis. Cambridge University Press, New York. Jeune, B., and Barabé, D. 2004. Statistical recognition of random and regular phyllotactic patterns. Ann. Bot. (Lond.), 94: 913–917. PMID:15477231. Jönsson, H., Heisler, M.G., Shapiro, B.E., Meyerowitz, E.M., and Mjolsness, E. 2006. An auxin-driven polarized transport model for phyllotaxis. Proc. Natl. Acad. Sci. U.S.A. 103: 1633–1638. doi:10.1073/pnas.0509839103. PMID:16415160. Kang, J., and Dengler, N. 2002. Cell cycling frequency and expression of the homeobox gene ATHB-8 during leaf vein development in Arabidopsis. Planta, 216: 212–219. doi:10.1007/s00425002-0847-9. PMID:12447534. Kang, J., and Dengler, N. 2004. Vein pattern development in adult leaves of Arabidopsis thaliana. Int. J. Plant Sci. 165: 231–242. doi:10.1086/382794. Kang, J., Tang, J., Donnelly, P., and Dengler, N.G. 2003. Primary vascular pattern and expression of ATHB-8 in shoots of Arabidopsis. New Phytol. 158: 443–454. doi:10.1046/j.1469-8137. 2003.00769.x. Kelly, W.J.,and Cooke,T.J.2003.Geometrical relationships specifying the phyllotactic pattern of aquatic plants. Am. J. Bot. 90: 1131–1143. Kirchoff, B.K. 1984. On the relationship between phyllotaxy and vasculature: a synthesis. Bot. J. Linn. Soc. 89: 37–51. Can. J. Bot. Vol. 84, 2006 Kwiatkowska, D. 1995. Ontogenetic changes of phyllotaxis in Anagallis arvensis L. Acta Soc. Bot. Pol. 64: 259–271. Larson, P.R. 1975. Development and organization of the primary vascular system in Populus deltoides according to phyllotaxy. Am. J. Bot. 62: 1084–1099. doi:10.2307/2442125. Larson, P.R. 1977. Phyllotactic transitions in the vascular system of Populus deltoides Bartr. as determined by 14C labeling. Planta, 134: 241–249. doi:10.1007/BF00384188. Larson, P.R. 1980a. Interrelations between phyllotaxis, leaf development and the primary–secondary transition in Populus deltoides. Ann. Bot. (Lond.), 46: 757–769. Larson, P.R. 1980b. Control of vascularization by developing leaves. In Control of shoot growth in trees. Edited by C.H.A. Little. Proceedings of the International Union of Forest Research Organizations. Maritimes Forest Research Centre, Fredericton. pp. 157–172. Larson, P.R. 1983. Primary vascularization and the siting of primordia. In The growth and functioning of leaves. Edited by J.E. Dale and F.L. Milthorpe. Cambridge University Press, Cambridge, UK. pp. 25–51. Lomax, T.L., Muday, G.K., and Rubery, P.H. 1995. Auxin transport. In Plant hormones: physiology, biochemistry, and molecular biology. Edited by P.J. Davies. Kluwer Academic Publishers, Dordrecht, Netherlands. pp. 509–530. Long, J., and Barton, M.K. 2000. Initiation of axillary and floral meristems in Arabidopsis. Dev. Biol. 218: 341–353. doi:10. 1006/dbio.1999.9572. PMID:10656774. Lough, T.J., and Lucas, W.J. 2006. Integrative plant biology: role of phloem long-distance macromolecular trafficking. Annu. Rev. Plant Biol. 57: 203–232. doi:10.1146/annurev.arplant.56. 032604.144145. PMID:16669761. Lyndon, R.F. 1990. Plant development — the cellular basis. UnwinHyman, London, UK. Lyndon, R.F. 1998. The shoot apical meristem — its growth and development. Cambridge University Press, Cambridge, UK. Marshall, C. 1996. Sectoriality and physiological organization in herbaceous plants: an overview. Vegetatio, 127: 9–16. doi:10. 1007/BF00054842. Mattsson, J., Chushumova, W., and Berleth, T. 2003. Auxin signaling in Arabidopsis leaf vascular development. Plant Physiol. 131: 1–13. McConnell, J.R., Emery, J., Eshed, Y., Bao, N., Bowman, J.L., and Barton, M.K. 2001. Role of PHABULOSA and PHAVOLUTA in determining radial patterning in shoots. Nature, 411: 709–713. doi:10.1038/35079635. PMID:11395776. Meinhardt, H. 1996. Models of biological pattern formation: common mechanisms in plant and animal development. Int. J. Dev. Biol. 40: 123–134. PMID:8735921. Mitchison, G.J. 1977. Phyllotaxis and Fibonacci numbers. Science, 196: 270–275. doi:10.1126/science.196.4287.270. Nägeli, C.W. 1858. Über das Wachsthum des Stammes und der Wurzel bei den Gefässpflanzen und die Anordnung der Gefässtränge im Stengel. Bei. Wissenschaft. Bot. 1: 1–156. Nelson, T., and Dengler, N.G. 1997. Leaf vascular pattern formation. Plant Cell, 9: 1121–1135. doi:10.1105/tpc.9.7.1121. PMID:12237378. Orians, C.M., and Jones, C.G. 2001. Plants as resource mosaics: a function model for predicting patterns of within-plant resource heterogeneity to consumers based on vascular architecture and local environment. Oikos, 94: 493–504. doi:10.1034/j.16000706.2001.940311.x. Paponov, I.A., Teale, W.D., Trebar, M., Blilou, I., and Palme, K. 2005. The PIN auxin efflux facilitators: evolutionary and functional perspectives. Trends Plant Sci. 10: 170–177. doi:10.1016/ j.tplants.2005.02.009. PMID:15817418. # 2006 NRC Canada Dengler Philipson, W.R., and Balfour, E.E. 1963. Vascular patterns in dicotyledons. Bot. Rev. 29: 382–404. Poethig, R.S. 1990. Phase change and the regulation of shoot morphogenesis in plants. Science, 250: 382–404. Prigge, M.J., Otsuga, D., Alonso, J.M., Ecker, J.R., Drews, G.N., and Clark, S.E. 2005. Class III homeodomain-leucine zipper gene family members have overlapping, antagonistic, and distinct roles in Arabidopsis development. Plant Cell, 17: 61–76. doi:10.1105/tpc.104.026161. PMID:15598805. Reinhardt, D. 2005. Phyllotaxis — a new chapter in an old tale about beauty and magic numbers. Curr. Opin. Plant Biol. 8: 487–493. doi:10.1016/j.pbi.2005.07.012. PMID:16054863. Reinhardt, D., and Kuhlemeier, C. 2002. Phyllotaxis in higher plants. In Meristematic tissues in plant growth and development. Edited by M.T. McManus and B.E. Veit. Sheffield Academic Press, Sheffield. pp. 172–212. Reinhardt, D., Mandel, T., and Kuhlemeier, C. 2000. Auxin regulates the initiation and radial position of plant lateral organs. Plant Cell, 12: 507–518. doi:10.1105/tpc.12.4.507. PMID:10760240. Reinhardt, D., Pesca, E., Stieger, P., Mandel, T., Baltenspergr, K., Bennett, M., Traas, J., Friml, J., and Kuhlemeier, C. 2003. Regulation of phyllotaxis by polar auxin transport. Nature, 426: 255–260. doi:10.1038/nature02081. PMID:14628043. Reinhardt, D., Frenz, M., Mandel, T., and Kuhlemeier, C. 2005. Microsurgical and laser ablation of leaf positioning and dorsiventral patterning in tomato. Development, 132: 15–26. PMID:15563522. Richards, F.J. 1951. Phyllotaxis: its quantitative expression and relation to growth in the apex. Philos. Trans. R. Soc. Lond. B Biol. Sci. 235: 509–564. Sachs, T. 1981. The control of patterned differentiation of vascular tissues. Adv. Bot. Res. 9: 151–262. Sachs, T. 1991. Cell polarity and tissue patterning in plants. Dev. Suppl. 1: 83–93. 1671 Scarpella, E., Francis, P., and Berleth, T. 2004. Stage-specific markers define early steps of procambium development in Arabidopsis leaves and correlate termination of vein formation with mesophyll differentiation. Development, 131: 3445–3456. doi:10.1242/dev.01182. PMID:15226260. Scarpella, E., Marcos, D., Friml, J., and Berleth, T. 2006. Control of leaf vascular patterning by polar auxin transport. Genes Dev. 20: 1015–1027. doi:10.1101/gad.1402406. PMID:16618807. Schwabe, W.W. 1984. Phyllotaxis. In Position controls in plant development. Edited by P.W. Barlow and D.J. Carr. Cambridge University Press, Cambridge, UK. pp. 403–440. Skipworth, J.P. 1962. The primary vascular system and phyllotaxis in Hectorella caespitosa Hook. N.Z. J. Sci. 5: 253–258. Smith, R.S., Guyomarc’h, S., Mandel, T., Reinhardt, D., Kuhlemeier, C., and Prusinkiewicz, P. 2006. A plausible model of phyllotaxis. Proc. Natl. Acad. Sci. U.S.A. 103: 1301–1306. Steeves, T.A., and Sussex, I.M. 1989. Patterns in plant development. Cambridge University Press, Cambridge, UK. Stieger, P.A., Reinhardt, D., and Kuhlemeier, C. 2002. The auxin influx carrier is essential for correct leaf positioning. Plant J. 32: 509–517. doi:10.1046/j.1365-313X.2002.01448.x. PMID:12445122. Vaughan, J.G. 1955. The morphology and growth of the vegetative and reproductive apices of Arabidopsis thaliana (L.), Capsella bursa-pastoris (L.) Medic., and Anagallis arvensis L. Bot. J. Linn. Soc. 55: 279–301. Williams, L., Grig, S.P., Xie, M., Christensen, S., and Fletcher, J.C. 2005. Regulation of Arabidopsis shoot apical meristem and lateral organ formation by microRNA miR166g and its AtHD-ZIP target genes. Development, 132: 3657–3668. doi:10.1242/dev. 01942. PMID:16033795. Zhong, R., Taylor, J.J., and Ye, Z.H. 1999. Transformation of the collateral vascular bundles into amphivasal vascular bundles in an Arabidopsis mutant. Plant Physiol. 120: 53–64. doi:10.1104/ pp.120.1.53. PMID:10318683. # 2006 NRC Canada