* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Molecular Approaches for the Analysis of Gene Structure and Function
Survey
Document related concepts
Transcript
Molecular Approaches for the Analysis of
Gene Structure and Function
1. Cardiac adrenoreceptors: model genes
2. Cultured primary cells: heart cells
3. Heterologous cultured cells: model system to study gene
function
4. Detection of expressed proteins: anti-sera to native protein or
epitope tagging
5. Detection of Protein-protein interactions: Immunoprecipitation,
BRET and yeast 2-hybrid
6. The use of Green Fluorescent protein (GFP)
7. RNAi technology: knock down of endogeneously expressed
genes
8. Animal models: transgenics and gene knockouts
1.Cardiac adrenoreceptors: model genes
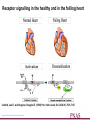
Receptor signalling in the healthy and in the failling heart
Limbird, Lee E. and Vaughan, Douglas E. (1999) Proc. Natl. Acad. Sci. USA 96, 7125-7127
Copyright ©1999 by the National Academy of Sciences
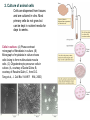
2. Culture of animal cells
Cells are dispersed from tissues
and are cultured in vitro. Most
primary cells do not grow but
can be kept in nutrient media for
days to weeks.
Cells in culture. (A) Phase-contrast
micrograph of fibroblasts in culture. (B)
Micrograph of myoblasts in culture shows
cells fusing to form multinucleate muscle
cells. (C) Oligodendrocyte precursor cells in
culture. (A, courtesy of Daniel Zicha; B,
courtesy of Rosalind Zalin; C, from D.G.
Tang et al., J. Cell Biol. 148:971 984, 2000)
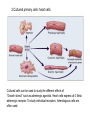
2.Cultured primary cells: heart cells
Cultured cells can be used to study the different effects of
“Growth stimuli” such as adrenergic agonists. Heart cells express all 3 Beta
adrenergic receptor. To study individual receptors, heterologous cells are
often used.
Cannot study individual
βARs in cultured heart
cells. Use heterologous
cells.
Iso (β-AR agonist) induces cardiomyocyte apoptosis. Cardiac myocytes were
incubated for 48 h with the indicated concentrations of Iso (0.1-50 µM) or 1 µM
ionomycin. The percentage of TUNEL-positive cardiomyocytes is presented as the
average ± S.E. from three independent experiments. *, p < 0.05 versus control.
J Biol Chem. 2000 Nov 3;275(44):34528-33
3.Heterologous
Cells
Some Commonly Used Cell Lines**
CELL LINE*
CELL TYPE AND ORIGIN
3T3
fibroblast (mouse)
BHK21
fibroblast (Syrian hamster)
MDCK
epithelial cell (dog)
HeLa
epithelial cell (human)
PtK1
epithelial cell (rat kangaroo)
L6
myoblast (rat)
PC12
chromaffin cell (rat)
SP2
plasma cell (mouse)
COS
kidney (monkey)
293
kidney (human); transformed with
adenovirus
CHO
ovary (chinese hamster)
DT40
lymphoma cell for efficient targeted
recombination (chick)
R1
embryonic stem cells (mouse)
E14.1
embryonic stem cells (mouse)
H1, H9
embryonic stem cells (human)
S2
macrophage-like cells (Drosophila)
**Many of these cell lines were derived from tumors. All of them are capable of indefinite replication in culture and
express at least some of the special characteristics of their cell of origin. BHK21 cells, HeLa cells, and SP2 cells
are capable of efficient growth in suspension; most of the other cell lines require a solid culture substratum in order to multiply.
Individual βAR can
be introduced into
cells and studied.
Introduction of DNA into animal cells A eukaryotic gene of interest is cloned in a
plasmid containing a drug resistance marker that can be selected for in cultured
animal cells. The plasmid DNA is introduced into cultured cells as a calcium
phosphate coprecipitate, which is taken up and expressed by a fraction of the cells
for a few days (transient expression). Stably transformed cells, in which the plasmid
DNA becomes integrated into chromosomal DNA, can be selected for by their ability
to grow in drugcontaining medium.
4. Detection of expressed Proteins: anti-sera to native protein
Purify protein
to use as
antigen
(or for
functional
assays)
Fusion proteins are often designed as immunogens for raising antibodies. In this
example, the plasmid vector has an origin of replication (ori) and an ampicillin resistance
gene (ampr) for growth in E. coli. The multiple cloning site (MCS) is located immediately
adjacent to a lacZ gene which can encode b-galactosidase with transcription occurring
from the lacZ promoter (PLAC) in the direction shown by the arrow. A cDNA sequence
from a gene of interest (gene X) is cloned in a suitable orientation into the MCS.
Expression from the lacZ promoter will result in a b-galactosidase-X fusion protein. This
can be used as an immunogen to raise antibodies to protein X. A popular alternative is to
use GST fusion proteins, where glutathione S transferase is coupled to the protein of
interest. The fusion protein can be purified easily by affinity chromatography using
glutathione agarose columns.
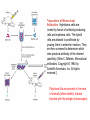
Preparation of Monoclonal
Antibodies. Hybridoma cells are
formed by fusion of antibody-producing
cells and myeloma cells. The hybrid
cells are allowed to proliferate by
growing them in selective medium. They
are then screened to determine which
ones produce antibody of the desired
specificity. [After C. Milstein. Monoclonal
antibodies. Copyright © 1980 by
Scientific American, Inc. All rights
reserved.]
Polyclonal Ab are present in the sera
of animals (often rabbits) that are
injected with the antigen (immunogen)
Western blotting Proteins are separated according
to size by SDS-polyacrylamide gel electrophoresis
and transferred from the gel to a filter. The filter is
incubated with an antibody directed against a protein
of interest. The antibody bound to the filter can then
be detected by reaction with various reagents, such
as a radioactive probe that binds to the antibody.
Detection of primary Ab with conjugated 20 Ab
Western blot analysis can be used to
determine the expression of specific proteins
Quantitation of the amount of ß1-, ß2-, and
ß3-AR in plasma membrane preparations
from control ( ), 14-week—STZ-induced
diabetic ( ), and 12-week—STZ-induced/2week—insulin-treated diabetic ( ) rat hearts.
Diabetes 50:455-461, 2001
4. (continued) Detection of expressed
Proteins: epitope tagging
Epitope tagged proteins can
be detected using Abs directed
against epitope. No need for Ab to
endogenous protein.
Epitope tagging allows the localization or purification of proteins. Using
standard genetic engineering techniques, a short epitope tag can be added to a
protein of interest. The resulting protein contains the protein being analyzed plus a
short peptide that can be recognized by commercially available antibodies. The
labeled antibody can be used to follow the cellular localization of the protein or to
purify it by immunoprecipitation or affinity chromatography.
Commonly used epitope tags
Sequence of tag
Origin
Location
mAb
DYKDDDDK
synthetic FLAG
N, C terminal
anti-FLAG M1
EQKLISEEDL
human c-Myc
N, C terminal
9E10
MASMTGGQQMG
T7 gene 10
N terminal
T7.Tag Ab
QPELAPEDPED
HSV protein D
C terminal
HSV.Tag Ab
RPKPQQFFGLM
substance P
C terminal
NC1/34
YPYDVPDYA
influenza HA1
N, C terminal
12CA5
5.Detection of Protein-protein interactions: Immunoprecipitation, BRET and 2-hybrid
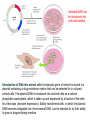
GPCR dimerization
Figure 1 | Role of homoand heterodimerization
in the transport of Gprotein-coupled
receptors. When
expressed alone, the
GABABR1 (GBR1) receptor
is retained as an immature
protein in the endoplasmic
reticulum (ER) of cells and
never reaches the cell
surface. By contrast, the
GBR2 isoform is
transported normally to
the plasma membrane but
is unable to bind GABA
and thus to signal. When
coexpressed, the two
receptors are properly
processed and transported
to the cell surface as a
stable dimer, where they
act as a functional
metabotropic GABAB
receptor.
Nat Rev Neurosci. 2001 Apr;2(4):274-86
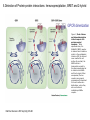
Figure 19.4.1 Flow
chart for the
coprecipitation of two
proteins that have
been differentially
tagged and
introduced into the
host organism. Ig h
and Ig l,
immunoglobulin
heavy and light
chains; NT, no tag.
Current Protocols in Protein Science
Published by John Wiley & Sons, Inc
Fig. 1. Principle of bioluminescence resonance energy transfer assay. Upper panels: Schematic
representation of seven transmembrane opioid receptors fused to Renilla luciferase (Rluc) and yellow
fluorescent protein (YFP). When they are far apart the light from the luminescent donor Rluc cannot excite
the fluorescent acceptor YFP (A). If two differentially tagged receptors interact and are brought close
together the acceptor is excited and emits light at 530 nm (B). Lower panels: Typical spectra obtained in the
absence (A) and in the presence (B) of receptor–receptor interactions with a peak at 470 nm (A) and peaks
at 470 and 530 nm (B).
The yeast two-hybrid system for detecting protein-protein interactions. The target
protein is fused to a DNA-binding domain that localizes it to the regulatory region of a
reporter gene as "bait." When this target protein binds to another specially designed
protein in the cell nucleus ("prey"), their interaction brings together two halves of a
transcriptional activator, which then switches on the expression of the reporter gene.
The reporter gene is often one that will permit growth on a selective medium. Bait and
prey fusion proteins are generated by standard recombinant DNA techniques. In most
cases, a single bait protein is used to fish for interacting partners among a large
collection of prey proteins produced by ligating DNA encoding the activation domain of
a transcriptional activator to a large mixture of DNA fragments from a cDNA library.
6. The use of Green Fluorescent protein (GFP)
GFP allows for the
detection of
protein
movement
within a cell
Schematic diagram representing key steps in GPCR signaling and
homologous desensitization. Note that the receptor gets internalized in an agonist
Dependant manner.
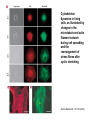
Translocation of ß-arrestin 2-GFP to the ß2-adrenergic receptor (ß2AR). HEK
293 cells stably overexpressing the ß2AR were transiently transfected with ßarrestin 2-GFP. The distribution of ß-arrestin 2-GFP fluorescence was
visualized by confocal microscopy before (−Iso) and after a 5 min treatment
with isoproterenol (+Iso; 10−8, 10−6 M) at 37 °C. Before agonist-stimulation, ßarrestin 2-GFP is uniformly distributed throughout the cytosol. Upon agonist
addition, ß-arrestin 2-GFP translocates from the cytosol to the plasma
membrane where it is found colocalizing with the receptor in punctuated areas
of the plasma membrane.
Cytoskeleton
dynamics in living
cells, as illustrated by
changes in the
microtubule and actin
filament network
during cell spreading
and the
rearrangement of
stress fibres after
cyclic stretching.
Nature Materials 2, 715–725 (2003)
7. RNAi technology: knock down of endogeneously expressed genes
The Mechanism of RNA Interference (RNAi)
RNAi allows the study of cells
lacking a specific protein (gene)
Introducing RNAi into cells.
RNAi
Ambion
Example of RNAi knock down.
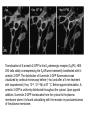
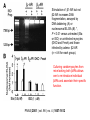
RNAi-mediated knockdown of Cav-1. A,
representative immunoblot (IB) of five clonally
isolated cell lines screened for the presence of
Cav-1 (equal amount of protein was loaded in
duplicates). B, representative immunoblot for
RNAi clone showing knockdown of protein Cav-1
with respect to wt C6 glioma cell lysates. Samples
were loaded in duplicate and also probed for total
G q and RSK1 to assess nonspecific knockdown
of unrelated proteins.
Knockdown of Cav-1 expression impairs
signaling of 5-HT2A receptors. For these
experiments wt C6 glioma cells and RNAimediated knocked down Cav-1 cells were plated
onto 96-well plates. Panel shows the sigmoid
dose response to serotonin (5-HT) in normalized
relative fluorescence units (RFU).
8. Animal models: transgenics and gene knock-outs
Gene replacement, gene knockout, and gene addition. A normal gene can be
altered in several ways in a genetically engineered organism. (A) The normal gene
(green) can be completely replaced by a mutant copy of the gene (red), a process
called gene replacement. This provides information on the activity of the mutant gene
without interference from the normal gene, and thus the effects of small and subtle
mutations can be determined. (B) The normal gene can be inactivated completely, for
example, by making a large deletion in it; the gene is said to have suffered a knockout.
(C) A mutant gene can simply be added to the genome. In some organisms this is the
easiest type of genetic engineering to perform. This approach can provide useful
information when the introduced mutant gene overrides the function of the normal
gene.
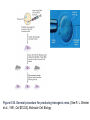
Figure 8-36. General procedure for producing transgenic mice. [See R. L. Brinster
et al., 1981, Cell 27:223.] Molecular Cell Biology
Summary of the procedures used for making
gene replacements in mice. In the first step (A),
an altered version of the gene is introduced into
cultured ES (embryonic stem) cells. Only a few
rare ES cells will have their corresponding normal
genes replaced by the altered gene through a
homologous recombination event. Although the
procedure is often laborious, these rare cells can
be identified and cultured to produce many
descendants, each of which carries an altered
gene in place of one of its two normal
corresponding genes. In the next step of the
procedure (B), these altered ES cells are injected
into a very early mouse embryo; the cells are
incorporated into the growing embryo, and a
mouse produced by such an embryo will contain
some somatic cells (indicated by orange) that carry
the altered gene. Some of these mice will also
contain germ-line cells that contain the altered
gene. When bred with a normal mouse, some of
the progeny of these mice will contain the altered
gene in all of their cells. If two such mice are in
turn bred (not shown), some of the progeny will
contain two altered genes (one on each
chromosome) in all of their cells. If the original
gene alteration completely inactivates the function
of the gene, these mice are known as knockout
mice. When such mice are missing genes that
function during development, they often die with
specific defects long before they reach adulthood.
These defects are carefully analyzed to help
decipher the normal function of the missing gene
Stimulation of β1-AR but not
β2-AR increases DNA
fragmentation, assayed by
DNA laddering (A) or
nucleosomal ELISA (B). *,
P < 0.01 versus untreated (Sta
or ISO) or uninfected myocytes
(DKO and Fresh) and those
infected by adeno- β2-AR
(n = 4-6 for each group).
Culturing cardiomyocytes from
mice lacking both βARs allows
one to re-introduce individual
βARs and ascertain their specific
function.
PNAS |2001 | vol. 98 | no. 4 | 1607-1612
Using DNA microarrays to monitor the expression of
thousands of genes simultaneously. To prepare the
microarray, DNA fragments each corresponding to a
gene are spotted onto a slide by a robot. Prepared
arrays are also available commercially. In this example,
mRNA is collected from two different cell samples for a
direct comparison of their relative levels of gene
expression. These samples are converted to cDNA and
labeled, one with a red fluorochrome, the other with a
green fluorochrome. The labeled samples are mixed and
then allowed to hybridize to the microarray. After
incubation, the array is washed and the fluorescence
scanned. In the portion of a microarray shown, which
represents the expression of 110 yeast genes, red spots
indicate that the gene in sample 1 is expressed at a
higher level than the corresponding gene in sample 2;
green spots indicate that expression of the gene is higher
in sample 2 than in sample 1. Yellow spots reveal genes
that are expressed at equal levels in both cell samples.
Dark spots indicate little or no expression in either
sample of the gene whose fragment is located at that
position in the array. For details see Figure 1-45.
(Microarray courtesy of J.L. DeRisi et al., Science
278:680 686, 1997. © AAAS.)