* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download The Drugs - chem4520
Pharmacokinetics wikipedia , lookup
Psychopharmacology wikipedia , lookup
Discovery and development of antiandrogens wikipedia , lookup
Discovery and development of cephalosporins wikipedia , lookup
Neuropharmacology wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Discovery and development of direct Xa inhibitors wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Prescription costs wikipedia , lookup
Drug interaction wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Discovery and development of proton pump inhibitors wikipedia , lookup
Drug design wikipedia , lookup
NK1 receptor antagonist wikipedia , lookup
Discovery and development of non-nucleoside reverse-transcriptase inhibitors wikipedia , lookup
Discovery and development of integrase inhibitors wikipedia , lookup
Pharmacognosy wikipedia , lookup
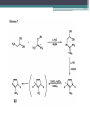
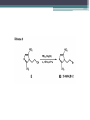
The Drugs Laura Duffin Introduction • Four different series of compounds were synthesized • Each compound had a phenanthridinium DNAtargeting moiety at one end • Active compounds also contained an electron affinic heteroaromatic moiety at the other end Series One • First series of compounds was prepared to test whether targeting to DNA changed the relationship between radiosensitizing ability and electron affinity Series Two • Second series examined the length of the spacer arm separating the DNA binding group from the active portion of the drug Series Three • Third series was designed to examine the properties of the DNA-binding group alone Series Four • Fourth series, non-radiosensitizing bioreductive agent, and the non-bioreductive, radiosensitizing nitrotriazole moiety Overall • Generally you tried to first attach an electrophilic spacer arm to the bioreduccible portion of the drug which was either purchased or synthesized from alicyclic precursors • Then functional group manipulation and phenanthridine was alkylated by the side chain Drugs Used • 2-Nitroimidazoles • 1-(2-Methyl-5-nitro-1H-imidazolyl)-2bromoethane • 4- and 5-Nitroimidazoles • 5-Nitrofurans • 3-Nitropyrroles • 1-Substituted-3- and 5-nitro-1,2,4-triazoles • N3-Substrituted-3-amino-1,2,4-triazine-1,4dioxides 2-Nitroimidazoles • Synthesis was tedious and expensive but could be scaled up to give the large quantities of the microbial agent required to make several different 2-nitroimidazole based compounds • Very good yield • The best yields and shorter reaction times were obtain when DMSO was used as a solvent 1-(2-Methyl-5-nitro-1H-imidazolyl)-2bromoethane • Using metronidazole treated with triphenylphosphine-Br2 in acetonitrile produced a yield of 95% but the triphenylphosphine could not be separated from the product using chromatography • Could have used extremely pyrophoric triethylphosphine to give a more easily separated oxide 4- and 5-Nitroimidazoles • It was found that 4-nitroimidazoles would be the favoured tautomer in polar solvents and 5nitroimidazole in the gas phase • Low yields were produced for both due to the decomposition caused by longer reflux periods 5-Nitrofurans • Convenient attachment of a spacer arm to the 5nitro-2-furoic acid • Phenanthridine was then added to the arm • Good yields 3-Nitropyrroles • Several routes of synthesis were tried but all of them showed unimpressive yields Summary of Yields Summary of Yields