* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Synaptogenesis and the Proteins that influence its Connectivity
Survey
Document related concepts
Transcript
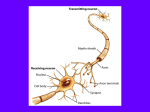
Synaptogenesis and the Proteins that influence its Connectivity By: Dominique Johnson Molecular Biology/ Biology 325 May 9, 2015 I hereby certify that this paper is free from plagiarism. I have documented all material, included all references used in the proper format, cited any paraphrased material or direct quotes, and enclosed direct quotes in quotation marks. I understand that violations of the plagiarism policy will result in referral to the Bridgewater College Honor Council and possible disciplinary action. Signed: Dominique Johnson One may realize that as they age their cognitive abilities have significantly improved over the years of their life. One is able to hold more information, pay more meaningful attention to their environment, and as an adult or an older child one is able to learn complex subjects much more quickly than that of an elementary school age child. This is all accomplished through a process called synaptogenesis, or the development of new synapses. This process occurs right after birth, infants develop new synapses at a rate of a million connections per second (1). Synapses are formed based off of a “use it or lose it” mentality. This means that if that connection is not used, it goes through a process called pruning which will get rid of the synapse. How then is it that some synaptic connections are better connected compared to others? The quality of a synaptic connection is based on the proteins that form that synapse. There are proteins that guide parts of the neuron to their specific destination allowing for adhesive proteins to hold the synapse together. These destinations include an axon to a postsynaptic neuron, muscle, or organ. Then there are the proteins that function within the synapse itself, such as SynCAM and neurotransmitters. The proteins that start synaptogenesis are Neuregulin and Nmethyle- D aspartate receptors, these are the proteins that guide the axon. (1, 2, 5, and 7). Neuregulin (NRG1) and N-methyl-D aspartate receptors (NMDAR) play important roles in various Neuronal functions. NRG1 is a transmembrane protein that is thought to regulate the function of NMDAR in cortical neurons. This protein is a member of the neuregulin family, which consist of four related genes (NGR1-4) (2). They are characterized by them all having an epidermal growth factor (EGF) that activates EGF- receptors – related tyrosine kinases (ErbB) (Osaki). NRG1 functions in migration of neurons, axon projection, myelination, and neurotransmitter maintenance (2). NMDARs promote the proliferation and survival of neuroblasts before synaptogenesis (2). The functions of NMDARs is determined by NR2 subunits (NR2A or NR2B) and NR2 is induced by NRG1. NMDARs main purpose is to stabilize synapses long term (3). They facilitate signaling pathways involved in neuronal development, such as learning and memory (2). Early NR2A expression blocks the addition of new spines in the neuron. Even when NR2B is added later it does not fix the number of synapse formed, that were significantly decreased due to the early expression of NR2A and its effects on spine formation. NR2B may contribute to the initiation of synapses, but without it, or with NR2A early expression, the setup is not prepared, thus fewer spines are formed to make synapses (4). Erbb4- Mutant mice, mice without the Erbb4 gene, research found that without this gene early brain development neurons cannot develop properly. For migration to occur NRG1 must be able to communicate with ErbB, or else neuronal development will stop. The communication between ErbB and NRG1 provide pattern information for the neurons to follow and enables them to form in the proper spot at the appropriate time during migration. NRG1 also plays a role in axon guidance, which allows a single neuron to grow outward rather than in a single direction. This process also consists of NRG1’s communication with ErbB (4). Specifically the thalamus cortical axon is dependent on NRG1 and ErbB. These axons are in charge of sending sensory and motor neurons to the cerebral cortex. Studies have shown that without NRG1 there is no way to guide the thalamus cortical axon to the cortex. NRG1 has been found to play a major role in the myelination of neurons. Glial cells, Schwann cells in the PNS and oligodendrocytes in the CNS, are the cells that actually make the myelin sheath around our neurons. Disrupting NRG1 signaling leads to almost a complete loss of Schwann cells. The purpose of myelin is to speed up the rate at which cells sends information to their destination (another neuron, muscle, or organ (2). Without myelination, the Central nervous system and the peripheral nervous system cannot fire information at a high rate. Brain derived neurotrophic factor (BDNF) is a mediator of synapse formation. It is a part of the neurotrophin family and is most active in the hippocampus (involved in learning and memory), because that is one of the few structures that produces new neurons throughout life. BDNF binds to TrkB, which is a type of tyrosine kinase that regulates synaptic strength, eliciting intracellular signaling pathways within the neuron. BDNF enhances NR2B mediated synaptic transmission by activating TrkB. TrkB form a ligand with BDNF and working together regulate plasticity. Like NRG1, BDNF also regulates neural development, while NMDARs regulates the neuronal functions before synaptogenesis. NRG1 works to induce NR2B activation in immature neurons by using BDNF/TrkB dependent mechanism. This means the TrkB is needed for NRG1 to activate NR2B before synaptogenesis (5). NMDARs are what signal BDNF, so that later they become the mechanisms that activate NMDAR. Studies show that without NMDAR there is a decrease in the amount of BDNF, thus a decrease in synapse production (5). A different protein that has not been discussed is DLG5 MAGUK, and it plays an important role in dendritic spine formation. The loss of DLG5 leads to a decrease in the number of spines that are formed in a dendrite and excitatory synapses. MAGUK regulates the localization of trans- synaptic cell adhesion. In the DLG family however there are PSD95 which are the subunits that control the localization and trafficking of glutamate receptor through direct interaction with NMDA. If PSD95 is missing or reduced it could lead to impaired spatial learning. This is because with decrease number of dendrites there cannot be as many synapses formed (6). Neurexins (Nrxn) and neuroligins (Nlgn) are adhesion molecules that connect the synapse thus allowing neurons to communicate. Nlgn are postsynaptic transmembrane proteins that are involved in binding the neuron with another neuron by forming a ligand with Neurexins (5). The Nlgn connect with a Nrxn using an enzyme called cholinesterase. There are four different types of Nlgn in primates, Nlgn 1 which is commonly used in excitatory synapses. There is also Nlgn 2 which is generally used in inhibitory synapses, Nlgn 3 which is involved in both inhibition and excitatory synapses. Then lastly Nlgn 4, this one’s function is unclear, but is speculated to involve glutamate synapses. Studies have been conducted on knockout (KO) mice, or mice who have had their DNA engineered to not express a specific gene. In Nlgn KO mice it has been shown that there are impairments in synaptic transmission. This is because Nlgn must form a connection with a presynaptic protein, like Nrxn. If the protein has been engineered to not be expressed in the mouse then the synapses that were supposed to form can no longer form (7). Some studies have link mutations in these proteins may be a result of autism (8). Neurexins are a single pass transmembrane presynaptic protein that involves the binding of two neurons by forming a ligand with Nlgn (8). In mammals there are three different types of Nrxn genes and each has a different promoter resulting in α (long forms) and β (short form) Nrxn. When Nrxn and Nlgn interact there is one Nlgn dimer that contacts with two monomeric Nrxn. Nlgn1 is the gene most likely to interact with Nrxn, which creates an excitatory synapse. These structures show that there was a presence of Ca2+ which explains the dependency binding has on Ca2+ . Ca2+ is essential for excitatory synapses to function (7). The interaction between Nlgn and Nrxn is called trans- synaptic, meaning that a post synaptic protein is interacting with a pre-synaptic protein. These two proteins working together are the two adhesive molecules that synapses need to form during synaptogenesis (5). They also deal with both excitatory and inhibitory synapses. In research done on removing all three α-Nrxn in KO mice it was found that their synaptic vesicle release was significantly lower than that of normal mice. This is due to the lack of Nrxn in inhibitory and excitatory synapses. Since Nrxn is involved in excitatory synapses one can assume that Nrxn may have the ability to interact with GABA. This interaction between Nrxn and GABA receptors is thought to work in cis (9). Nrxn is also known to communicate with SynCAMs As mentioned earlier, Nrxn not only communicate with Nlgn they also interact with SynCAMs. This protein is present on both post and presynaptic neurons. This protein even belongs to the same family as Nrxn and Nlgn, the immunoglobulin superfamily and it also participates in adhesive interactions and contributes to holding Axon and dendrite connection together; it is an adhesion molecule that directly impacts the number of synapses formed by altering the shape and growth of the receptors (10). SynCAM is involved in axo-dentritic interactions, meaning that it is involved in the communication between the axon of one cell and the dendrites of another. Synaptic adhesion by SynCAM 1 can increase the number of synapses formed all the while decreasing the plasticity of the neuron. High of this amounts of this protein allow for more synapses to be made during development. SynCAM is mostly involved in excitatory synapses in developing and fully developed brains (7). However, more synapses restrict the amount of plasticity within the synapses and neurons themselves. SynCAM KO mice showed improved spatial memory. In human, if there is a mutation in SynCAM or Nlgn then that person will be affected if autism (11). As one can see synaptogenesis is accomplished through a number of mechanisms that work together to form the long lasting connections that we have today. Some mechanisms been around since we were infants. One can also see that if one of these important mechanisms is mutated or purposely taken out (KO mice) for experimental reason it could have horrendous effects on the organism. Humans the lack Nrxn are typically diagnosed with autism, which could lead to future cognitive impairments. Without SynCAM synaptic connections could decrease all together which would have dastardly effects on one’s cognitive ability. Most individuals who lack any of these proteins often do not make it out of the embryonic stage. Those that do survive the embryonic stage and become an infant often times have learning impairments. Missing these proteins is far more detrimental than an infant losing part of their brain. The plasticity of the brain is at its highest between one and four meaning that if one hemisphere is removed (often a procedure for those with epilepsy) they would still be able to function because the neurons intended for one function can change into neurons intended for a different function. However, missing these proteins is a potential cause for many mental disabilities (1, 2, and 3) Works Cited 1. Levine E. Laura, Munsch, Joyce. Child Development an Active Learning Approach. SAGE Publications. 2011. 2. Mei L, Xion WC. Neuregulin 1 in Neural Development Synaptic Plasticity and Schizophrenia. Naturals Reviews Neuroscience. 2008. 9:437-452. 3. Grambill, Abigail C. NMDA receptor subunit composition controls synaptogenesis and synapse stabilization. Proceedings of the National Academy of Sciences of the United States of America. 2011. 108:14:5855-5860. 4. Pandya C, Pillai A. TrkB interacts with ErbB4 and regulates Nrg1-inducded Nr2B Phosphorylation in Cortical Neurons before synaptogenesis. BioMed Central 2014, 12:41 5. Runkel F, Rohlmass A, Reissner C, Brand SM, Missler M. Promoter- like Sequences Regulating Transcriptional Activity in Neurexin and Neuroligin Genes. Journal of Neurochemistry. 2013. 127:36-47. 6. Wang SH, Celic I, Choi SY, Riccomagno M, Wang Q, Sun Lu, Mitchell SP, Vasioukhin V, Huganir RL, Koldkin AL. Dlg5 Regulates Dendritic Spine Formation and Synaptogenesis by Controlling Subcellular N-Cadherin Localization. Journal of Neuroscience. 2014. 34(38):12745-12761. 7. Wright G, Washbourne Philip. Neurexins, Neuroligins, and LRRTMs: Synaptic Adhesion Getting Fishy. Journal of Neuroscience. 2011. 117:765-778. 8. Robbins E, Krupp A, Arce K, Ghosh A, Fogel A, Bourcard A, Sudhof T, Stein V, Biederer T. SynCAM 1 Adhesion Dynamically regulates Synapse Number and Impacts Plasticity and Learning. Neuron. 2010. 68:894-906. 9. Ren Zhen, Sahir Nadia, Murakami S, Luellen B, Earnheart J, Lal R, Kim JK, Luscher B. Defects in Dendrite and Spine Maturation and Synaptogenesis Associated with an Anxious- Depressive- Like Phenotype of GABAA Receptor- Deficient Mice. Neuropharmacology. 2015. 171-179. 10. Stagi Massimilliano, et. Al. SynCAM 1 participates in Axo- dendritic Contact Assembly and shapes Neuronal Growth Cones. 2010. 107-16. 11. Zhiling, Yu, et.al. Mutations in the gene encoding CADM1 are associated with autism spectrum disorder. 2008. 10-107.