* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter 6 - Section 1-The Chemical Context of Life
Survey
Document related concepts
Transcript
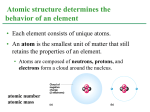
Chapter 6 The Chemical Context of Life PowerPoint® Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from Joan Sharp Copyright © 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings Overview: A Chemical Connection to Biology Biology is a multidisciplinary science Living organisms are subject to basic laws of physics and chemistry Example: Ants - Species Myrmelachista o Kill non-host trees by injecting formic acid into their leaves o They maintain an environment that their colony can survive and thrive Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings Life can be organized into a different structural levels. Organism Organ System Tissues Cellular Molecular Atomic At each level additional distinct properties appear. Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings Matter consists Elements & Compounds Organisms are composed of matter Matter is anything that takes up space and has mass Matter is made up of elements and compounds ELEMENTS & COMPOUNDS Element: a substance that cannot be broken down into other substances by chemical reactions. Compound: is a substance consisting of two or more elements in a fixed ratio o A compound has characteristics different from those of its elements Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings E.g. Table Salt Sodium Chlorine Sodium chloride Essential Elements of Life About 25 of the 92 elements are essential to life 96% of living matter Carbon Hydrogen Oxygen Nitrogen Remaining 4% Phosphorus, Sulfur, Calcium, Potassium, Others Trace elements are those required by an organism in minute quantities Iodine & Iron Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings Fig. 2-4 (a) Nitrogen deficiency (b) Iodine deficiency Concept 2.2: Structure of An Atom Each element consists of unique atoms Atom: Smallest unit of matter that still retains the properties of an element Atoms are composed Neutrons (no electrical charge) Protons (positive charge) Electrons (negative charge) Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings Cloud of negative charge (2 electrons) Atom Proton Charge +ve Neutron Neutral Electron -ve Electrons Nucleus Properties Stays the same in an element & determines element Varies in an element Determine chemical properties & reactivity of an element Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings Atomic Number and Atomic Mass Atoms of the various elements differ in number of subatomic particles Atomic number: Number of protons in its nucleus Mass number: Sum of protons plus neutrons in the nucleus Atomic mass: The atom’s total mass, can be approximated by the mass number Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings Isotopes All atoms of an element have the: Same number of protons But may differ in number of neutrons Isotopes: Are two atoms of an element that differ in number of neutrons Radioactive Isotopes: Decay spontaneously, giving off particles and energy Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings Some applications of radioactive isotopes in biological research are: Dating fossils Tracing atoms through metabolic processes Diagnosing medical disorders PET Scan – Detects location of intense chemical activity Pt. injected with glucose labeled with radioactive carbon Particles collide with electrons from chemical reactions in the body The PET detects these hot spots” Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings Chemical Bonds Interactions between usually result in atoms staying close together, held by attractions called chemical bonds Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings Covalent Bonds A covalent bond is the sharing of a pair of electrons by two atoms Covalent bonds can form between atoms of the same element or atoms of different elements In a covalent bond, the shared electrons count as part of each atom’s valence shell Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings Fig. 2-11 Hydrogen atoms (2 H) Hydrogen molecule (H2) A molecule consists of two or more atoms held together by covalent bonds A compound is a combination of two or more different elements A single covalent bond, or single bond, is the sharing of one pair of valence electrons A double covalent bond, or double bond, is the sharing of two pairs of valence electrons Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings Ionic Bonds Atoms sometimes steal electrons from their bonding partners An example is the transfer of an electron from sodium to chlorine After the transfer of an electron, both atoms have charges Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings Ionic Bonds A charged atom (or molecule) is called an ion Those that gain electrons become negatively charged Those that lose electrons are positively charged Na Na Sodium atom Cl Cl Chlorine atom Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings Na Cl Cl– Na+ Chloride Sodium ion ion (an anion) (a cation) Sodium chloride (NaCl) A Cation is a positively charged ion An Anion is a negatively charged ion An Ionic bond is an electrical attraction between an anion and a cation Compounds formed by ionic bonds are called ionic compounds, or salts Ionic Bonds are weak Salts, such as sodium chloride (table salt), are often found in nature as crystals Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings Fig. 2-15 Na+ Cl– Hydrogen Bonds A hydrogen bond forms when a hydrogen atom covalently bonded to one electronegative atom is also attracted to another electronegative atom In living cells, the electronegative partners are usually oxygen or nitrogen atoms Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings Fig. 2-16 + Water (H2O) + Hydrogen bond Ammonia (NH3) + + + Van der Waals Interactions • If electrons are distributed asymmetrically in molecules or atoms, they can result in “hot spots” of positive or negative charge • Van der Waals interactions are attractions between molecules that are close together as a result of these charges Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings • Collectively, such interactions can be strong, as between molecules of a gecko’s toe hairs and a wall surface Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings You should now be able to: 1. Identify the four major elements 2. Distinguish between the following pairs of terms: neutron and proton, atomic number and mass number, atomic weight and mass number 3. Distinguish between and discuss the biological importance of the following: nonpolar covalent bonds, polar covalent bonds, ionic bonds, hydrogen bonds, and van der Waals interactions Copyright © 2008 Pearson Education, Inc., publishing as Benjamin Cummings