* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download PPT File
Survey
Document related concepts
Transcript
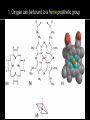
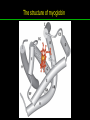
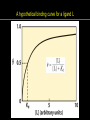
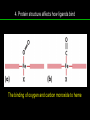
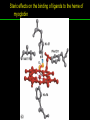
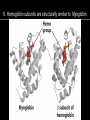
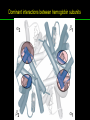
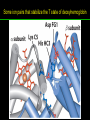
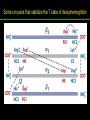
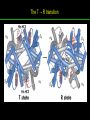
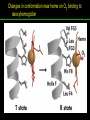
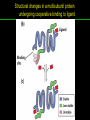
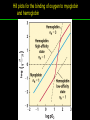
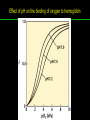
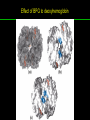
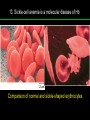
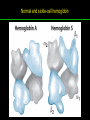
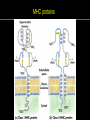
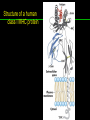
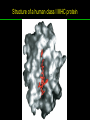
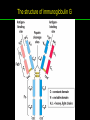
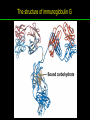
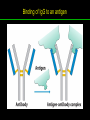
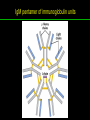
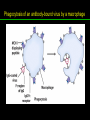
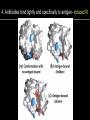
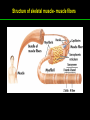
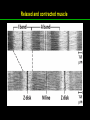
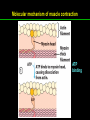
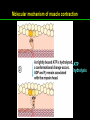
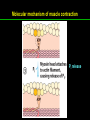
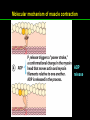
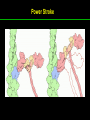
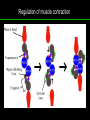
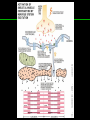
高等生化學 Advanced Biochemistry Protein Function 陳威戎 Key principles of protein function 1. The functions of many proteins involve the reversible binding of other molecules. 2. A ligand binds at a site on the protein called the binding site, which is complementary to the ligand. 3. Proteins are flexible. 4. The binding of a protein and ligand is often coupled to a conformational change. 5. Interactions between ligands and proteins may be regulated. Protein Function 1. Reversible Binding of a Protein to a Ligand: Oxygen-Binding Proteins 2. Complementary Interactions between Proteins and Ligands: The Immune System and Immunoglobulins 3. Protein Interactions Modulated by Chemical Energy: Actin, Myosin, and Molecular Motors I. Reversible Binding of a Protein to a Ligand: Oxygen-Binding Proteins 1. Oxygen can be bound to a heme prosthetic group. 2. Myoglobin has a single binding site for oxygen. 3. Protein-ligand interactions can be described quantitatively. 4. Protein structure affects how ligands bind. 5. Oxygen is transported in blood by hemoglobin. 6. Hemoglobin subunits are structurally similar to myoglobin. 7. Hemoglobin undergoes a structural change on binding oxygen. 8. Hemoglobin binds oxygen cooperatively. 9. Cooperative ligand binding can be described quantitatively. 10. Two models suggest mechanisms for cooperative binding. 11. Hemoglobin also transports H+ and CO2. 12. Oxygen binding to hemoglobin is regulated by 2,3-BPG. 13. Sickle-cell anemia is a molecular disease of hemoglobin. 1. Oxygen can be bound to a heme prosthetic group 2. Myoglobin has a single binding site for oxygen. The structure of myoglobin 3. Protein-ligand interactions can be described quantitatively Equilibrium expression of the reversible binding of a protein (P) to a ligand (L): Ka: association constant 3. Protein-ligand interactions can be described quantitatively Kd: dissociation constant A hypothetical binding curve for a ligand L A curve describing the binding of oxygen to myoglobin Some Protein Dissociation Constants 4. Protein structure affects how ligands bind The binding of oxygen and carbon monoxide to heme Steric effects on the binding of ligands to the heme of myoglobin 5. Oxygen is transported in blood by hemoglobin 1. Erythrocytes (red blood cells) 2. Hemocytoblasts 3. Saturation of oxygen in arterial and venous blood 4. Myoglobin (Mb): insensitive to small changes in O2 conc. oxygen storage 5. Hemoglobin (Hb): highly sensitive, oxygen transport 6. Hemoglobin subunits are structurally similar to Myoglobin Amino acid sequence comparison for Mb, Hba and Hbb Dominant interactions between hemoglobin subunits 7. Hemoglobin undergoes a structural change on binding O2 1. Two major conformations of hemoglobin: R (relaxed) state and T (tense) state 2. T state is more stable (deoxyhemoglobin) 3. T state is stabilized by a greater number of ion pairs, many of which lie at the a1b2 (and a2b1) interface. 4. Binding of O2 to a Hb subunit in the T state triggers a change in conformation to the R state, narrowing the pocket between the b subunits. Some ion pairs that stabilize the T state of deoxyhemoglobin Some ion pairs that stabilize the T state of deoxyhemoglobin The T → R transition Changes in conformation near heme on O2 binding to deoxyhemoglobin 8. Hemoglobin binds O2 cooperatively 1. Hb must bind O2 efficiently in the lungs (pO2= 13.3 kPa), and release O2 in the tissues (pO2= 4 kPa). 2. Hb solves the problem by undergoing a transition from a lowaffinity state ( the T state) to a high-affinity state ( the R state) as more O2 molecules are bound, 3. Hb has a hybrid S-shaped, or sigmoid, binding curve for O2. 4. Allosteric protein: binding of a ligand to one site affects the binding properties of another site on the same protein. 5. Modulators: inhibitors or activators; homotropic or heterotropic A sigmoid (cooperative) binding curve Structural changes in a multisubunit protein undergoing cooperative binding to ligand Structural changes in a multisubunit protein undergoing cooperative binding to ligand 9. Cooperative ligand binding can be described quantitatively Equilibrium expression of a protein (P) with n binding sites to a ligand (L): Ka: association constant Hill equation nH: Hill coefficient ; a measure of the degree of cooperativity Hill plots for the binding of oxygen to myoglobin and hemoglobin Carbon monoxide: a stealthy killer Carbon monoxide: a stealthy killer 10. Two models suggest mechanisms for cooperative binding Concerted model (MWC model): proposed by Jacques Monod, Jefferies Wyman, and Jean-Pierre Changeux in 1965 Sequential model: proposed by Daniel Koshland and colleagues in 1966 Two general models for the interconversion of inactive and active forms of cooperative ligand-binding proteins 11. Hemoglobin also transports H+ and CO2 Hb carries two end products of cellular respiration - H+ and CO2 – from the tissues to the lungs and the kidneys. A reaction catalyzed by carbonic anhydrase. (in erythrocytes) The binding of O2 by Hb is profoundly influenced by pH and CO2 concentration. ~ Bohr effect Hb transports about 40% of the total H+ and 15-20% of the CO2. In peripheral tissues, low pH and high [CO2] → O2 released In the capillaries of the lung, high pH and low [CO2] → O2 bound 11. Hemoglobin also transports H+ and CO2 In Hb, O2 binds to the iron atom of the hemes. H+ binds to specific amino acid residues. CO2 binds as a carbamate to the a-amino group at the Nterminal end of each globin chain. Effect of pH on the binding of oxygen to hemoglobin 12. O2 binding to Hb is regulated by BPG The interaction of 2,3-bisphosphoglycerate (BPG) with Hb provides an example of heterotropic allosteric modulation. BPG is known to greatly reduce the affinity of Hb for O2. BPG binds at a site distant from the O2binding site and regulates the O2- binding affinity of Hb in relation to the pO2 in the lungs. Effect of BPG on the binding of oxygen to hemoglobin Effect of BPG to deoxyhemoglobin 13. Sickle-cell anemia is a molecular disease of Hb Comparison of normal and sickle-shaped erythrocytes Normal and sickle-cell hemoglobin Normal and sickle-cell hemoglobin A single a.a. substitution: Glu6 to Val6 in two b chains HbS has two fewer negative charges than HbA. Creates a “sticky” hydrophobic contact point on the outer surface, causes deoxyHbS to associate abnormally with each other, forming the long, fibrous aggregates. II. Complementary Interactions between Proteins and Ligands: The Immune System and Immunoglobulins 1. The immune response features a specialized array of cells and proteins. 2. Self is distinguished from nonself by the display of peptides on cell surfaces. 3. Antibodies have two identical antigen-binding sites. 4. Antibodies bind tightly and specifically to antigen. 5. The antibody-antigen interaction is the basis for a variety of important analytical procedures. 1. The immune response features a specialized array of cells and proteins. 2. Self is distinguished from nonself by the display of peptides on cell surfaces. MHC (major histocompatibility complex) proteins Class I MHC Each individual produces up to 6 class I MHC variants. Bind and display peptides derived from cellular proteins. Recognition targets of the T-cell receptors of the TC cells. Class II MHC Occur on the surfaces of macrophages and B lymphocytes. Each human produces up to 12 variants. Bind and display peptides derived from external proteins. Recognition targets of the T-cell receptors of the TH cells. MHC proteins Structure of a human class I MHC protein Structure of a human class I MHC protein 3. Antibodies have two identical antigen-binding sites. Five classes of immunoglobulins: 5 types of heavy chain: a, d, e, g, m ; 2 types of light chain: k and l IgD, IgE: overall structures similar to that of IgG IgE: allergic response; interacts with basophils and mast cells. IgM: a cross-linked pentamer, first Ab made by B lymphocytes, major Ab in the early stages of primary immune response. IgA: found in secretions such as saliva, tears, and milk, can be a monomer, dimer or trimer. IgG: major Ab in secondary immune responses, initiated by memory B cells ; most abundant Ab in the blood. The structure of immunoglobulin G The structure of immunoglobulin G Binding of IgG to an antigen IgM pentamer of immunoglobulin units Phagocytosis of an antibody-bound virus by a macrophage 4. Antibodies bind tightly and specifically to antigen- induced fit 5. The antibody-antigen interaction is the basis for a variety of important analytical procedures. Two types of antibodies preparations are in use: Polyclonal antibodies Monoclonal antibodies: by Köhler and Milstein, 1975 Practical uses of antibodies: Affinity column ELISA (enzyme-linked immunosorbent assay) Immunoblot assay (Western blot) Antibody techniques- general method Antibody techniques- ELISA III. Protein interactions modulated by chemical energy: Actin, myosin, and molecular engineering 1. The major proteins of muscle are myosin and actin. 2. Additional proteins organize the thin and thick filaments into ordered structures. 3. Myosin thick filaments slide along actin thin filaments. Motor Proteins underlies~ 1. Contraction of muscles Myosin ; Actin ; additional proteins 2. Migration of organelles along microtubules Kinesins ; Dyneins 3. Rotation of bacterial flagella Rotary motor complex (Proton turbine) 4. Movement of some proteins along DNA Helicases, polymerases, etc. 1. The major protein of muscle are myosin and actin-Myosin Myosin (Mr 540,000): 6 subunits 2 Heavy chains (each of Mr 220,000) 4 Light chains (each of Mr 20,000) C-terminus: extended a helices wrapped around each other in a fibrous, left-handed coiled coil similar to a-keratin. N-terminus: ATP-binding sites; light chains associated with the globular domains Myosin has two heavy chains and two light chains Myosin has two heavy chains and two light chains Cleavage with trypsin and papain separates the myosin heads from the tails Ribbon representation of myosin S1 fragment The major components of muscle- myosin Thick filament 1. The major protein of muscle are myosin and actin-actin G-actin (globular actin; Mr 42,000): monomeric actin F-actin (filamentous actin): long polymer of G-actin Thin filament: F-actin + troponin + tropomyosin The major components of muscle- F-actin Thin filament Actin filament bound with myosin head Structure of skeletal muscle- muscle fibers Relaxed and contracted muscle 2. Additional proteins organize the thin and thick filaments into ordered structures. Minor muscle proteins in thin filaments: a-actinin ; desmin ; vimentin ; nebulin Minor muscle proteins in thick filaments: paramyosin ; C-protein ; M-protein ; titins Molecular rulers that regulates the length of the thin and thick filaments: nebulin and titin. Titin extends from the Z disk to the M line. 2. Additional proteins organize the thin and thick filaments into ordered structures. Muscle contraction Sarcomere- Contracting 3. Myosin thick filaments slide along actin thin filaments. Molecular mechanism of muscle contraction Molecular mechanism of muscle contraction ATP binding Molecular mechanism of muscle contraction ATP hydrolysis Molecular mechanism of muscle contraction Pi release Molecular mechanism of muscle contraction ADP release Power Stroke Regulation of muscle contraction Interactions between actin and myosin must be regulated so that contraction occurs only in response to appropriate signals form the nervous system. Regulation is mediated by a complex of two proitens: tropomyosin: binds to the thin filament, blocking the attachment sites for the myosin head groups. troponin: a Ca2+-binding protein, causes a conformational change in the complex, exposing the myosin-binding sites on the thin filaments. Regulation of muscle contraction Regulation of muscle contraction