* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Pituitary adenomas in adolescence: diagnostic approach
Hormone replacement therapy (male-to-female) wikipedia , lookup
Hypothalamus wikipedia , lookup
Signs and symptoms of Graves' disease wikipedia , lookup
Neuroendocrine tumor wikipedia , lookup
Hyperandrogenism wikipedia , lookup
Kallmann syndrome wikipedia , lookup
Growth hormone therapy wikipedia , lookup
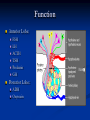
Pituitary adenomas in adolescence: diagnostic approach and therapeutic strategy 4° Joint Meeting on Adolescence Medicine Catanzaro, 14-16 October 2010 Piernicola Garofalo Graziella Malizia U.O.C Endocrinologia A.O.O.R. Villa Sofia-Cervello Palermo Pituitary adenomas Pituitary adenomas are rarely diagnosed in childhood and adolescence, but their mass effect and endocrine abnormalities can compromise both quality and length of life. Many signs or symptoms of pituitary adenoma, complained of in adulthood, not became evident during adolescence, suggesting true prevalence of this tumor in teenagers is higher than expected. Pituitary adenoma occuring during adolescence are associated with features or therapeutic needs sometimes different from those occuring in adulthood. At the onset of disease, delay in growth was rarely observed in teenagers with pituitary adenomas. Many girls complain of oligoamenorrhoea and galactorrhoea, while headache and delay in pubertal development are the most commons features in boys. Hypopituitarism is occasionally encountered in adolescence. Early diagnosis and appropriate choice of therapy are necessary to avoid permanent endocrine complications of disease and its treatment. Pituitary adenomas The estimated incidence of pituitary adenoma in children is still unknown since most published series included patients with onset of symptoms before the age of 20 years as pediatric patients. Pituitary adenomas constitute less than 3% of supratentorial tumors in children and 2.3-6% of all pituitary tumors treated surgically. The average annual incidence of pituitary adenoma in children has been estimated to be 0.1/million. Pituitary carcinomas are rare in adults and extremely rare in children In children, more frequently than in adults, pituitary tumors may be a manifestation of genetic conditions such as multiple endocrine neoplasia type 1 (MEN 1), Carney complex, familial isolated pituitary adenoma (FIPA), and McCune-Albright syndrome. Pituitary adenomas Prolactinoma is indeed the most frequent adenoma histotype in children, followed by the ACTHoma and the somatotropinoma. Non-functioning pituitary adenomas, TSH-secreting, and gonadotropin-secreting adenomas are very rare in children accounting for only 3-6% of all pituitary tumors. ACTH secreting adenomas have earlier onset and predominate in the prepubertal period. GH secreting adenomas are very rare before puberty. Similar to adults, presenting symptoms are generally related to the endocrine dysfunction rather than to mass effect. Function Anterior Lobe: FSH LH ACTH TSH Prolactin GH Posterior Lobe: ADH Oxytocin Prolactin secreting adenomas Prolactinomas are the most frequent tumors both in childhood and in adulthood, and their frequency varies with age and sex, occurring more frequently in females (until 42-68 % in differents study). Prolactinomas are usually diagnosed at the time of puberty or in the postpubertal period and clinical manifestations vary in keeping with the age and sex of the child. Although growth arrest is typically seen in children and adolescents before ephiphyseal fusion is completed. Pre-pubertal children generally present with a combination of headache, visual disturbances, growth failure. Growth failure is not a common symptom. Impairment of other pituitary hormone secretion was found only in a minority of patients (27%). Spontaneous or provoked galactorrhea is seen in 50% of children. Prolactin secreting adenomas Macroadenomas at presentation are more likely in boys than in girls. Young hyperprolactinemic men were shown to have a more severe impairment of BMD than patients in whom hyperprolactinemia occurred at an older age. In another series of patients, occurrence of hyperprolactinemia during adolescence has a lower BMD than those having adult onset tumors Females may present with pubertal delay, amenorrhea, and other symptoms of hypogonadism. In males, macroprolactinomas are more frequent; accordingly, males with prolactinomas also have a higher incidence of neurological and opthalmological abnormalities (i.e. cranial nerve compression, headaches, visual loss), growth or pubertal arrest and other pituitary dysfunctions. Prolactin secreting adenomas Treatment strategy In the absence of complications needing immediate surgery, such as visual loss, hydrocephalus or cerebrospinal fluid leak, pharmacotherapy with dopamine agonists should be considered the first treatment approach. In children, cabergoline (CBA) has been used successfully by several investigators at doses starting at 0.25 mg twice weekly and ranging from 0.5-3.5 mg/week orally. The easily weekly administration makes CAB an excellent therapeutic approach to children . Prolactin secreting adenomas “Clinical tips” Hyperprolactinemic children expressed a wide variety of initial clinical presentations. The most common were growth and puberty disorders and obesity. PRL determination should be included in investigation protocols of obese and short stature children. Horm. Res. Paediatr. 2010;73(3):187-92. ACTH-secreting adenomas Between 11 and 15 years of age, ACTH secreting adenomas are the most frequent cause of adrenal hyperfunction and the second most frequent pituitary adenoma after prolactinomas. A macroadenoma is rarely the cause of Cushing's disease (CD) in children. The clinical manifestations of CD are mostly the consequence of excessive cortisol production. The clinical presentation is highly variable The diagnosis is generally delayed since a decrease in growth rate may be the only symptom for a long time. Growth failure in CD may be due to a decrease of free IGF1 levels and/or a direct negative effect of cortisol on the growth plate. In children with CD, the direct negative effect of hypercortisolism on bone formation is further worsened by concomitant hypogonadism and GH deficiency, both of which are associated with decreased BMD. ACTH-secreting adenomas Children with CD may have impaired carbohydrate tolerance, while overt diabetes mellitus is uncommon. Excessive adrenal androgens may cause acne and excessive hair growth, or premature sexual development in the first decade of life. Hypercortisolism may cause pubertal delay in adolescent patients. Peculiarly, young patients with CD may present neuropsychiatric symptoms which differ form those of adult patients. Frequently they tend to be obsessive and are high performers at school. The differential diagnosis of CD includes adrenal tumors, ectopic ACTH production, and ectopic CRH producing tumors. However, ectopic ACTH secretion is extremely rare in the pediatric age. ACTH-secreting adenomas Diagnosis In a child/adolescent with suspected CD the diagnosis is based on measurement of levels of cortisol and ACTH. Measurement of 24-h urinary free cortisol is elevated, and a low dose of dexamethasone (15 µg/Kg) at midnight does not induce suppression of morning serum cortisol concentrations as in normal subjects. Suppression of the spontaneous circadian variations of serum cortisol is another feature of CD. Suppression of cortisol by more than 50% after high dose dexamethasone (150 µg/Kg) given at midnight will confirm that hypercortisolism is due to an ACTH-secreting pituitary adenoma ACTH-secreting adenomas Differential diagnosis of Cushing’s disease ACTH-secreting adenomas Different aetiologies of paediatric CS from the literature (n= 398 cases) shown at ages of peak incidence Storr et al. Trends Endocrinol Metab 2006;18:167-174 ACTH-secreting adenomas Treatment strategy Trans sphenoidal adenomectomy is the treatment of choice for ACTH secreting adenomas. Surgical remission is successful in the majority of children, with initial remission rates of 7098% and long term cure of 50-98% in most studies. Surgery is usually followed by adrenal insufficiency and patients require hydrocortisone replacement for a few months. After normalization of cortisol levels, resumption of normal growth or even catch up growth can be observed. Generally, final height is compromised compared to target weight. ACTH-secreting adenomas “Clinical Tips” The management of pediatrics CD patients after cure also presents challenges for optimizing growth, bone health, reproduction and composition from childhood into and during adult life. Pituitary. 2007;10(4):365-71 Growth hormone secreting adenomas In childhood, GH-secreting adenomas account for 5-15% of all pituitary adenomas. In less than 2% of the cases excessive GH secretion may depend on a hypothalamic or ectopic GH releasing hormone (GHRH) - producing tumor (gangliocytoma, bronchial or pancreatic carcinoid), which causes somatotroph hyperplasia or a well-defined adenoma. Chronic GH hyper secretion is characterized by local bone overgrowth, while in children and adolescents it leads to gigantism because of the associated secondary hypogonadism which delays epiphyseal closure, thus allowing continued bone growth. All growth parameters are affected although not necessarily symmetrically, mild to moderate obesity occurs frequently, and macrocephaly has been reported. In girls, menstrual irregularity can be present while glucose intolerance and diabetes mellitus are rare. Growth hormone secreting adenomas Diagnosis The diagnosis is usually clinical, and can be readily confirmed by measuring GH levels, which in more than 90% of patients are above 10 mg/l. The OGTT is the simplest and most specific dynamic test for both the diagnosis and the evaluation of the optimal control of GH excess. IGF1 values should be referred to pubertal stage. Treatment strategy The objectives of treatment of GH excess are tumor removal with resolution of its eventual mass effect, restoration of normal basal and stimulated GH secretion, relief of symptoms directly caused by GH secretion, relief of symptoms directly caused by GH excess and prevention of progressive disfigurement, bone expansion, osteoarthritis and cardiomyopathy which are disabling long-term consequences, as well as prevention of hypertension, insulin resistance, lipid abnormalities that are risk factors for vascular damage. Surgery, radiotherapy and pharmacological suppression of GH levels are the currently available treatment options Growth hormone secreting adenomas Treatment strategy Transphenoidal adenomectomy remains the first treatment for GHsecreting tumors. Currently, cure criteria are serum GH levels below 2.5 mg/l, glucose suppressed GH levels below 1 mg/l together with age-normalized IGF-1 levels. Treatment with somatostatin analogs is very effective in patients with GH hypersecretion, although few data in adolescent patients have been reported. Promising new therapeutic agents have recently emerged in the form of competitive GHRH and GH antagonists which have been shown to effectively suppress GH and/or IGF-1 levels. TSH-secreting adenomas This tumor type is rare in very rare in childhood and adolescence with only a few cases reported so far. It is frequently a macroadenoma presenting with mass effect symptoms such as headache, visual disturbances, together with variable signs and symptoms of hyperthyroidism. It must be differentiated from the syndrome of thyroid hormone resistance . Treatment strategy TSS is the first treatment approach to these tumors. In adults, radiotherapy is recommended as routine adjunctive therapy. However, due to high frequency of post-radiotherapy hypopituitarism, in children pharmacotherapy is the preferred second choice. Chronic treatment with SR-lanreotide reduced plasma TSH and normalized fT4 and fT3 levels, suggesting its use in the long-term medical treatment of these adenomas Nonfunctioning adenomas FSH and LH-secreting tumors with a clinical picture of hormone hypersecretion are very rare. The majority of FSH/LH-producing adenomas are clinically asymptomatic. In pediatric patients they represent account for less than 4-6% of cases. The clinical presentation included visual field defects, headache and some degree of pituitary insufficiency since invariably all patients had macroadenoma. In the pediatric population, these adenomas need to be differentiated from other sellar/parasellar masses such as cysts, craniopharyngioma and dysgerminoma. Therefore, the MRI of the sella and parasellar structure is the basic step in the diagnosis. Nonfunctioning adenomas Treatment strategy The first approach to these adenomas is trans sphenoidal surgery (TSS) to remove tumor mass and decompress parasellar structures. After surgery, these patients partially recover from hypopituitarism. Postoperative radiotherapy is applied in patients with subtotal tumor removal to prevent tumor regrowth and reduce residual tumors, but is burdened by high prevalence of panhypopituitarism. Positive effects of cabergoline were observed in some patients with a subunit secreting adenomas, mostly in patients with tumor expressing high number of dopamine D2 receptors. Craniopharyngiomas Craniopharyngiomas comprise the majority (80 to 90%) of neoplasms found in the pituitary fossa of children: up to 15% of all intracranial tumors in childhood are craniopharyngiomas. These tumors have a bimodal age-specific incidence: they occur most frequently at age 5 to 14 years and rarely in the fifth decade of life. Incidence does not vary with gender or race. Most craniopharyngiomas (70% of the total) give symptoms they are extended in both the intrasellar and suprasellar regions; 30% of the tumors may be either intra- or suprasellar in location. Craniopharyngiomas typically present with endocrine dysfunction, decreased vision, and an intense headache or other symptoms related to increased intracranial pressure. By histology, these tumors are benign; however, craniopharyngiomas can behave aggressively through papillae that invade surrounding bony structures and tissues. In addition, they can have cystic components that may enlarge and compress adjacent structures. Case report 1 G.L. female patient was born on 26/9/74 Menarche at 13 years with regular menses until May 1994, since then amenorrhea, six months after treated with estro-progestogens. November '95: persists amenorrhea and galactorrhea is reported that both spontaneous and provoked is hospitalized: PRL: 186 ng/ml. Perform brain MRI pituitary: “…extensive process to expansive character with prevailing intra-and suprasellar location, roughly oval, with diameter. max of about 17 x18x15, that appears to occupy the spaces aditus sellae, but with expanded storage of the profile of dorsun sellae and extends to occupy the upper reservoir space Ferner and characterized extensively the optic chiasm, located up ... do not recognize, in relation to ' extension of the expansion process, the pituitary stalk…” Case report 1 February ‘96: Begin therapy with 10 mg/day of bromocriptine. Perform ophthalmologic examination and campimetric, results were normal. April ’96: PRL: 101-105 ng/ml December ’96: PRL: 120-122 ng/ml August '97: resumption of menses with oligomenorrhea Case report 1 June '97: MRI brain-pituitary "gland morphofunctional alteration with presence in the right half of the hypointense area of basic weights and which becomes, after contrast medium, even more markedly hypointense with respect to the remaining gland ... This lesion (significantly reduced volume than '95) leads to a slight imprint on the sellar floor and the tank overflows cranially sovrasellare right and may be attributable to regression to the area of necrosis of the previous macroadenoma. There is a modest shift of the pituitary stalk to the left and a portion of the lesion, which manifests itself above the carotid siphon signal that has typical adenomatous tissue. Not invasion of cavernous sinuses”. Case report 1 January 98: PRL: 83 ng/ml. Stopped bromocriptine and started cabergoline 1.5 mg/w. October 99: MRI brain pituitary "gland volume in the standard. Persistence of areola mildly hypointense on T1 on the right for possible persistence of residual adenomatous and/or residue regressive“. Case report 1 December ’99: PRL: 17,4 ng/ml March 2001: PRL: 17,3 July 2000: PRL: 21,3 Maj 2001: PRL: 19,6 Gennuary 2002: PRL: 17,3 February 2003: PRL: 13,3 October 2003: PRL: 28,4 March 2005: PRL: 8-8,5-8,9 Case report 1 October 2005: MRI brain-pituitary: "... gland volume in the physiological range, with only a minimal persistence of altered signal areola, subtle hypointense ... Case report 1 December 2006: PRL: 12,2 December 2007: PRL: 9,3 December 2008: PRL: 4,4 July 2010: PRL: 3,1 ng/ml. Echocardiography were normal. Continued cabergoline therapy. Pituitary MRI: Sellar normal volume. Case report 2 M.G. female patient was born on 03/04/1996, Target 157 cm, birth weight 3.050 kg, the age of 4 years early isolated pubarche. Due to a marked increase in weight (+ 10 kg), was referral for clinical evaluation. October 2004: H 128.7 cm (51 centile), weight 45 kg, BMI: 27.1. Facio-truncal. Obesity. Achantosis nigricans in the neck and roots of the limbs. PH3/B1 pubertal stage. ACTH at 8: 19 pg/ml, at 20: 7.6, Cortisol at 8: 24 mcg/dl, at 20: 13, after over night suppression test with dex: 2.8, after simple suppression test : 2.7, CLU: 400 mcg/24 h (88-359). 17OHP basal and after Synacthen, total and free testosterone, LH, FSH, E2, thyroid hormones, pelvic ultrasound: normal for age. IRI after OGTT in 60 'peak of 345.6 mcU / ml. Rx left hand and wrist: bone age equivalent to 8 years. January 2005: ACTH at 8: 32, 20:34, cortisol 8: 23,4, 20: 37, CLU: 549 , cortisol after over night suppression test: 17,24 mcg/dl CT adrenal glands: normal Case report 2 January 2005: MRI pituitary: "... no images certanly to refer to adenoma". February 2005: cortisol: 8: 33,5, CLU: 285-335 -484 CRF Test: ACTH: 30-113-95-60-39-32, cortisol: 35,8-44,2-47,4-56,2-48,437,8. Dex test, Cortisol: 4,5, dex 4 days cortisol: 1,8. February 2005: MRI pituitary (Niguarda): "... the adenohypophysis has a concave profile, the pituitary stalk median ... after MDC welcomes small and heterogeneous hypointensity of the right half of the gland, not surely due to pituitary adenoma. CT thorax: normal. March 2005: TNS surgery. Immunohistochemistry: ACTH positive, some cells positive for GH, PRL negative proliferative index (KI67/MIB-1) <1%. Start therapy Cortone acetate at a dose of 25 mg ½ + ¼ + ¼ H 128.3 cm, weight 45 kg, circumference 79 cm away. PH4/B2 pubertal stage. Case report 2 April 2005: H 130.9 cm, 42.8 kg, LH, FSH, E2, PRL, TSH: normal. IGF1: 345. June 2005: stop taking cortone. H 134.5 cm (55th percentile), kg 40.800, BMI 22.6, waist circumference 70 cm, vc: 10.9 cm/year. October 2005: CLU: 36 nmol/L, ACTH: 13 Cortisol: 12,5 February 2006: H 137.7 cm, kg 41.800, waist 71cm, FT4, TSH, PRL normal, ACTH at 8: 13, Cortisol 8: 9,46, CLU: 66, bone age 10.5 years. March 2006: MRI brain-pituitary: "... regular morphology saddled with reduced expression of pituitary asymmetrical right half of the gland with asymmetric depth of the tank-type configuration with sovrasellar aracnoidocele. Pituitary stalk in line…” Case report 2 March 2006: MRI brain-pituitary Case report 2 July 2006: H 140.8 cm (69th percentile), 44.600 kg, BMI: 22.5, waist circumference 71 cm, PH4/B3, vc: 7.5 cm/year. Menarche. November 2006: H 143.3 cm (71th percentile), kg 51.700, BMI: 25.2, waist circumference 74 cm, vc: 6.6 cm/year PH5/B4. ACTH: 6.8, Cortisol: 15, CLU: 67, oligomenorrhea January 2008: H 148.1 cm (46th percentile), kg 57.700, BMI: 26.3, waist circumference 75 cm, vc: 3.1 cm/year. Eumenorrea/oligomenorrhea. IGF1, LH, FSH, E2, TSH normal. ACTH at 8: 30 pg/ml, at 20: 34. Cortisol, 8: 5.7 ug/dl (1-39), 20: 4.9 (3-18), fasting IRI: 13 nU/ml, July 2009: H 148.8 cm (5th centile), kg 58.800, BMI 26.6, waist circumference 67.5 cm. Hirsutism from about 6 months, IFG: 13. Complained of aheadache. ACTH 8: 28, cortisol 8: 14.2, cortisol after over night suppression test: 6.1, LH, FSH, E2, PRL, total testosterone, free testosterone, DHEAS: normal, androstenedione: 3.1 ng/ml (0.3 to 3). Case report 2 August 2009: MRI Brain-pituitary: “… Recurrent microadenoma the left half of the pituitary gland…” Case report 2 January 2010: H 148.8 cm, kg 51.200, waist circumference 62 cm, ACTH 8: 64 pg/ml, 20: 73.2, Cortisol at 8: 10.67 ug/dl, 20: 13.6, CLU: 340,2 nmol/24h (38-208), cortisol after over night suppression test: 8.28 March 2010: MRI Brain-pituitary ... marked reduction in thickness with a small focal area of signal CSF dependent adenoipophysis right half as a result of surgery, the left half of the gland is appreciated with a profile along the top and also the dynamic study with contrast medium allows detection of hypointense parenchymal inhomogeneity of 0.5 cm… pituitary microadenoma. Small deviation to the right side of the pituitary stalk ... Case report 2 March 2010: Brain-pituitary MRI Case report 2 April 2010: TNS endoscopic surgery: removal of diseased tissue, white, soft laterosellare sn, pituitary gland observed. Immunohistochemistry: fragments of parenchyma pituitary FSH + LH + + GH, PRL + ACTH +, Ki67 (MIBI): <1%. May 2010: CLU: 434 mcg/24 h (28-213), ACTH: 25.3, cortisol: 8: 34, 20: 16. May 2010: TNS surgery by removal of some small fragments of pituitary parenchyma and fibrous tissue. Immunohistochemistry: fragments of parenchyma pituitary FSH + LH + + GH, PRL + ACTH + July 2010: CLU: 876.5, ACTH: 49.2, cortisol 8: 22, 20: 35. What therapy? First-line treatment for Cushing disease in childhood is always surgical; transsphenoidal adenomectomy or hemihypophysectomy in situations where the surgical exploration is negative has been shown to be nearly 90% curative. Radiation or gamma-knife therapy is reserved for these patients in whom surgical intervention failed. Bilateral adrenalectomy may be considered for inoperable or recurrent cases; however it is associated with a significant risk of development of Nelson's syndrome. Or ? What is Pasireotide (SOM230) ? Grazie per l’attenzione