* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download NOVEL FAK-SELECTIVE INHIBITORS
Development of analogs of thalidomide wikipedia , lookup
Discovery and development of HIV-protease inhibitors wikipedia , lookup
Discovery and development of dipeptidyl peptidase-4 inhibitors wikipedia , lookup
Discovery and development of direct Xa inhibitors wikipedia , lookup
Discovery and development of cyclooxygenase 2 inhibitors wikipedia , lookup
Discovery and development of antiandrogens wikipedia , lookup
Neuropharmacology wikipedia , lookup
Drug discovery wikipedia , lookup
Bevacizumab wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
MTOR inhibitors wikipedia , lookup
Theralizumab wikipedia , lookup
Discovery and development of integrase inhibitors wikipedia , lookup
Discovery and development of neuraminidase inhibitors wikipedia , lookup
Metalloprotease inhibitor wikipedia , lookup
Discovery and development of ACE inhibitors wikipedia , lookup
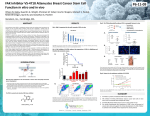
CRT OPPORTUNITY Small Molecules - In Vivo Proof of Principle NOVEL FAK-SELECTIVE INHIBITORS • Potent, highly selective and orally bioavailable compounds • Sustained modulation of PD biomarker and in vivo efficacy in combination with Avastin in TNBC • Potential to increase efficacy of PD-1 and CTLA-4 checkpoint inhibitors • Potential to reduce cancer stem cell burden THERAPEUTIC RATIONALE FAK is a non-receptor tyrosine kinase which is overexpressed (through either gene amplification or increased levels of protein expression), or over-activated in many human cancers (including solid tumours and acute myelogenous leukaemia (AML)). Increased levels of FAK and/or phosphorylated FAK are often associated with late stage metastatic, high grade tumours and poor patient outcome. FAK has been shown to play an important role in tumour cell growth and development, including promotion of cancer cell invasion and metastasis, angiogenesis, evasion of apoptosis, and acquisition of resistance to targeted and chemotherapeutic therapies. Through targeting FAK kinase activity in both the tumour and stroma, FAK inhibitors have potential utility in various cancer types to reduce primary tumour growth and metastatic disease (alone and in combination with current anti-cancer therapeutic regimens). Immuno-oncology: A new immuno-suppressive role for nuclear FAK has recently been reported, with nuclear FAK inhibiting cytotoxic CD8+ T cell activity via regulation of chemokine transcription and promotion of regulatory T cell (Treg) recruitment [1]. As such, there is rationale for combining FAK-selective inhibitors with checkpoint blockade therapies targeting PD-1 and CTLA-4. Indeed, this is supported by recent reports of a FAK/PYK2 inhibitor showing efficacy in vivo in combination with anti-PD-1 antibody [2] or anti-PD-1 antibody/anti-CTLA-4 antibody [3]. Non-cancer therapeutic utilities: FAK inhibitors also have potential utility in pulmonary fibrosis. POTENT AND SELECTIVE FAK INHIBITORS Novel competitive inhibitors with low nM in vitro activity against FAK have been developed. The lead compound CTx0294945 displays drug-like physiochemical properties and is a highly selective orally bioavailable FAK inhibitor with excellent in vitro efficacy and modulation of a pharmacodynamic (PD) biomarker (FAK autophosphorylation, pY397), good in vitro ADME properties and in vivo pharmacokinetic profile (Table 1). CTx-0294945 is highly selective over the related kinase PYK2, and against a wider panel of enzymes representing a spectrum of the kinome. Oral administration of CTx-0294945 (but not PF-562,271, another FAK inhibitor) gives sustained modulation of the PD biomarker pY397-FAK 12 hours following a final dose in xenografts (Figure 1). Moreover CTx-0294945 anti-tumour efficacy has been observed in combination with Avastin in the orthotopic TNBC model MDA-MB-231, where there is a decrease in the rate of tumour growth following cessation of treatment with the anti-angiogenic VEGFRa-trapping antibody Avastin (Figure 2) together with evidence of delayed tumour revascularisation and a trend to increased tumour growth inhibition. CTx-0294945 is differentiated from the clinical candidates of the competition by being highly selective and of distinct chemical structures together with low potential for drug-drug interactions. Cancer Stem Cells: FAK has also been identified as a key signalling target in cancer stem cell (CSC) survival [4-5], and FAK inhibitors shown to result in inhibition of CSC self renewal in vitro [6] and reduction in CSCs in tumours in vivo [7]. As such, FAK inhibitors have potential utility in various cancer types to reduce cancer stem cell burden, including cancers of high unmet medical need known to be enriched for CSCs (e.g. breast). Read more overleaf CRT OPPORTUNITY Small Molecules - In Vivo Proof of Principle CTx-0294945 Biological activity FAK Biochemical IC50 (nM) 2.2 PYK2 Biochemical IC50 (nM) 550 Cellular efficacy IC50 (nM) 63 (migration); 214 (MDA-231-LNA proliferation -3DoT assay) FAK PD biomarker activity IC50 (nM) - inhibition of Y397 - FAK phosphorylation 7 Rat pharmacokinetics Bioavailability (%F) 58% Half life (hr) 5.1 P450 IC50 (uM) >10 at all isoforms tested Glutathione trapping None Toxicity Well tolerated in multiple in vivo studies as single agent and in combination Table 1: Lead compounds properties Figure 2: Co-dosing with a FAK inhibitor significantly reduces the rate of tumour re-growth following suspension of Avastin treatment in MDA-231-LNA orthotopic model. (Black=vehicle; Red=CTx-0294945 single agent 80 mg/kg QD; Blue=Avastin single agent 12.5 mg/Kg twice-weekly; Purple=CTx-0294945 plus Avastin (doses as above)). ORIGINATORS These programmes originate from CRT’s Australian Partner Consortium, Cancer Therapeutics CRC (CTx). CTx is a dedicated cancer drug discovery company underpinned by public/private partnership funds. CTx is located in Melbourne, Australia, and works closely with leading Australian researchers and institutes to discover and develop small molecule discoveries into leads and preclinical drug candidates with commercial potential. CRT is CTx’s commercialisation partner for this project. INTELLECTUAL PROPERTY The intellectual property package includes a patent portfolio protecting the lead series and surrounding chemical space (WO2012/110774). Figure 1: Sustained in vivo target (FAK) engagement with CTx- 0294945. pY397-FAK levels in MDA-231-LNA orthotopic primary tumours were determined 12 hours after the last dose of drug. *p<0.05 relative to vehicle. COMMERCIAL OPPORTUNITY Cancer Research Technology is offering prospective commercial partners global therapeutic rights to the FAKselective inhibitor programme on an exclusive basis. REFERENCES 1. 2. 3. 4. 5. Serrels A et al., Cell. 2015. 163L160-173. Ring JE et al., AACR-NCI-EORTC 2015. Jiang H et al., AACR-NCI-EORTC 2015 Guan J et al., IUBMB Life 2010 62(4):268-276. Schober M and Fuchs E. Proc. Natl. Acad. Sci. USA 2011. 108(206):10544-10549. 6. Xu Q et al., AACR 2012 poster 7. Kolev VN et al., SABCS 2012 CONTACT Tanya Moore, Business Development Executive [email protected] +44 (0)20 3469 8700