* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Photon polarization wikipedia , lookup
History of electromagnetic theory wikipedia , lookup
Magnetic monopole wikipedia , lookup
Electromagnetism wikipedia , lookup
Electrical resistivity and conductivity wikipedia , lookup
Aharonov–Bohm effect wikipedia , lookup
Field (physics) wikipedia , lookup
Lorentz force wikipedia , lookup
Maxwell's equations wikipedia , lookup
Circular dichroism wikipedia , lookup
Atomic theory wikipedia , lookup
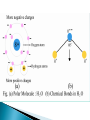
UNIT-II A medium plays a significant role in determining the response of an electric field. Depending upon the behavior of different materials in an electrostatic field, they are divided in two main categories: 1. Conductors 2. Insulators or dielectrics. Conductors are materials which contain electrons that are free to move and an electric field produces a steady drift of charge i.e. current. They “conduct” the current or allow the flow of electric charges like electrons fairly easily. Dielectric is basically an electrically insulating material. The electrons are strongly bound to the atoms or molecules. They cannot be separated by the application of an electric field. A steady flow of electrons cannot flow through it. The electric moment of a system of charges with zero net charge is generally called the electric dipole moment of the system. m aQ the vector m points from the negative to the positive charge. +Q a rp - Q rn Dielectric materials are classified into two broad groups: 1. Polar 2. Non-polar Polar molecule The molecules in which the arrangement or geometry of the atoms is such that one end of the molecule has a positive electrical charge and the other side has a negative charge. The centers of gravity of the positive and negative charge distributions do not coincide. They have a finite electric dipole moment (permanent dipole moment). E.g. H2O, NH3, HCl, SO2, H2S, CO. + - Non-polar molecule A non-polar molecule is that in which the electrons are distributed more symmetrically It does not have an excess /abundance of charges at the opposite sides. The centers of gravity of the positive and negative charge distributions coincide. They do not have any permanent dipole moment E.g. CO2, H2, N2, O2, CH4, CCl4 + - In polar dielectric molecules, permanent dipoles are present however, due to thermal agitation, they are generally in random orientations Dielectric is in unpolarized condition i.e. the net dipole moment within any small volume is zero. A dielectric possess no free electrons to provide currents due to an applied external E.F. Although there is no macroscopic migration of charge when a dielectric is placed in an electric field, microscopic displacements (on the order of the size of atoms or molecules) of charge occur resulting in the appearance of induced electric dipoles. There are two mechanisms of polarization: …displacement of charge within the atom or molecule …orientation of a molecule with a permanent dipole moment. A dielectric is said to be polarized when induced electric dipoles are present. The presence of induced electric dipoles within the dielectric causes the electric field to be modified. Polarizability is a measure of the ability of a material to become polarized in the presence of an applied electric field. Polarization occurs in both polar and non polar materials. electron cloud nucleus In the absence of an applied electric field, the positively charged nucleus is surrounded by a spherical electron cloud with equal and opposite charge. The dipole moment is zero. Eapp In the presence of an applied electric field, the electron cloud is distorted such that it is displaced in a direction (w.r.t. the nucleus) opposite to that of the applied electric field. e e p E dipole moment (C-m) polarizability (F-m2) The net effect is that each atom becomes a small charge dipole which affects the total electric field inside the material. In polar dielectric molecules, permanent dipoles are present however, due to thermal agitation, they are generally in random orientations Dielectric is in unpolarized condition i.e. the net dipole moment within any small volume is zero. Eapp In the presence of an applied electric field, the dipoles tend to align themselves with the applied electric field. When an electric field is applied, the dipoles are oriented by rotation and aligned in the direction of the electric field one type of bound charges (+ve or -ve) appear on a surface and the opposite type on opposite surface. e e p E dipole moment (C-m) polarizability (F-m2) The net effect is that each polar molecule is a small charge dipole which aligns with the applied electric field and influences the total electric field. A neutral atom + - R Polarized atom - E0 E + r X-axis Consider an atom of radius R having spherical symmetric charge distribution. (non-polar atom with zero dipole moment) If atom is subjected to an external E.F., both positive and negative charges will experience force in opposite directions. As positive charge is rigidly fixed in nucleus, its position remains fixed whereas negative charge is pushed along negative x-axis. Therefore atom acquires an induced dipole moment. p=Qe=Ze r Also net E.F. inside dielectric: Enet E0 E The force on nucleus due to applied electric field is F ZeE0 ZeE0iˆ There will be another force on nucleus due to negative charge cloud which will tend to restore original configuration. The charge on negative cloud is Q Ze The E.F. due to this cloud at the nucleus will be: E Qr Zer ˆ i 3 3 40 R 40 R The force on nucleus due to the charged cloud is: 2 2 ' Z e r ˆ F ZeE i 40 R 3 At equilibrium the total force on nucleus must be zero. F F' 0 2 2 Z er ˆ ˆ ZeE0i i 0 3 40 R 40 R 3 E0 r Ze but p Zer p 40 R 3 E0 p E0 This shows that the induced dipole moment is proportional to external E.F. Atomic polarizability: As p 40 R 3 E0 Let 40 R 3 p E0 where is called Atomic polarizabi lity. It is defined as induced dipole moment per unit E.F. p E0 For a given value of E0, larger the value of , larger will be induced dipole moment. Also, direction of induced dipole moment is same as external E.F. p E0 - E0 E p + X-axis If a sample of dielectric contain a N atoms per unit volume of the sample. Let p be the dipole moment induced in each atom. Then polarization produced in the sample is P Np NE0 N is the number of dipoles per unit volume. p is the average dipole moment of the (m-3) dipoles in the medium: Coulomb-m (C-m) is the atomic polarizability of the dipoles in the medium: Farad-m2 (F-m2) P is the polarization per unit volume: Coulomb- m-2 (C/m2) The charges which are developed on the surfaces of dielectric during polarization are called bound charges. As these charges are attached with atoms of material, they can’t take part in conduction. The bound charges are also called polarization charges. These charges are denoted by qb or q p All charges except produced by polarization are called free charges. A parallel-plate capacitor consists of two oppositely charged, conducting parallel plates separated by a finite distance. The field between the plates is uniform in direction (perpendicular to the plates) and magnitude. Charge density E.F. q/ A E0 q / o A / o If a dielectric slab is placed within capacitor plates, then dielectric gets polarized. As a result of polarization, the E.F. inside dielectric become E E0 EP where E P is E.F. produced by polarization charges. The dielectric constant of a material is defined as the ratio of E.F. inside the capacitor without dielectric to E.F. inside same capacitor with dielectric. k E0 / E E0 /( E0 EP ) but E P P / 0 k P For vacuum, P =0. Therefore k=1 The polarization of the dielectric is caused by the E.F. which causes alignment of dipoles. Therefore polarization should depend on net E.F. i.e. polarization is directly proportional to the net E.F. PE P 0 e E electron susceptibility (dimensionless) For vacuum, there is no polarization. Thus susceptibility of vacuum is zero. Under the influence of this field, the positive and negative charges in the particle are moved apart: the particle is polarized. In general, these induced dipoles can be treated as ideal; permanent dipoles, however, may generally not be treated as ideal when the field at molecular distances is to be calculated. The values of molecular dipole moments are usually expressed in Debye units. The Debye unit, abbreviated as D, equals 10-18 electrostatic units (e.s.u.). The permanent dipole moments of non-symmetrical molecules generally lie between 0.5 and 5D. It is come from the value of the elementary charge eo that is 4.410-10 e.s.u. and the distance s of the charge centers in the molecules amount to about 10-9-10-8 cm. In the case of polymers and biopolymers one can meet much higher values of dipole moments ~ hundreds or even thousands of Debye units. To transfer these units to CI system one have to take into account that 1D=3.3310-10 coulombsm. A volume distribution of dipoles may be represented as an equivalent volume (qevb) and surface (qesb) distribution of bound charge. These charge distributions are related to the dipole moment distribution: qevb P qesb P nˆ 33 Gauss’s law in differential form in free space: 0 E qev Gauss’s law in differential form in dielectric: 0 E qev qevb 34 0 E qev qevb qev P 0 E P qev • Hence, the displacement flux density vector is given by D 0 E P 35 Gauss’s law in differential form: Gauss’s law in integral form: D qev D d s Q encl S 36 C D 3 m Ddv dv v v D ds Q s (C ) E 0 Gauss’s Law: The total outward the dielectric displacement (or the outward flux) over any surface is equal to the total free enclosed in the surface flux of simply closed charge D 0 E P 0 E 0 e E C 0 (1 e ) E 0 r E E 2 m Where- ε is the absolute permittivity (F/m) -εr is the relative permittivity or the dielectric cons of the medium -ε0 is the permittivity of free space -χe is the electric susceptibility (dimensionless) Assuming that we have P 0 e E Dis the e permittivity E E or the The parameter 0 1electric dielectric constant of the material. 39 The concepts of polarizability and dipole moment distribution are introduced to relate microscopic phenomena to the macroscopic fields. The introduction of permittivity eliminates the need for us to explicitly consider microscopic effects. Knowing the permittivity of a dielectric tells us all we need to know from the point of view of macroscopic electromagnetics. 40 For the most part in macroscopic electromagnetics, we specify the permittivity of the material and if necessary calculate the dipole moment distribution within the medium by using P D 0 E 0 E 41 The relative permittivity of a dielectric is the ratio of the permittivity of the dielectric to the permittivity of free space r 0 42 3-6.2 Dielectric in static electric field Bound charges (electric dipole) : External electric field causes a force to be exerted on each charged particle and results in a small displacements of positive and negative charges in opposite directions. (fig. 3-14) The induced electric dipoles modify the electric field both inside and outside the dielectric material. Polar molecules possess permanent electric dipole moment. Polarization vector: the volume density of electric dipole moment. n P lim 0 p k 1 k (C/m 2 ). 3-7 Electric flux density and dielectric constant Divergence modified to include the effect of equivalent polarization volume charge density : EE 1 ( p ) 1 ( P P) or 0 0 (( 0 E P ) . Electric flux density (electric displacement) : D 0E P New equation : (C/m 2 ). D (C/m3 ), where : volume density of free charges. Generalized Gauss's law: DDd d , or D ds Q (C). D ds Q (C ) V V' C C The total outward flux of the electric displacement over any closed surface is equal to the total free charge enclosed in the surface. 3-7 Electric flux density and dielectric constant For linear and isotropic medium, P 0 eE, where e : electric susceptibility. Electric displacement : D 0 E P 0 E 0 e E 0 (1 e )E 0 r E E where r 1 e (C/m 2 ), : relative permittivity (dielectric constant), 0 0 r : absolute permittivity (permittivity). A simple medium: a linear, homogeneous, and isotropic medium. Anisotropic materials: the dielectric constant is different for different directions of the electric field. Dielectric constants and dielectric strengths of some common materials 3-7.1 Dielectric strength Dielectric strength: the maximum electric field intensity that a dielectric material can withhold without breakdown. Dielectric strength of air: 3 kV/mm. Electric field intensity at a charged conductor surface is higher at points of larger curvature.