* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download What are carboxylic acids?
Survey
Document related concepts
Transcript
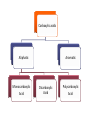
What are carboxylic acids? • Carboxylic acids are compounds which contain a Carboxyl group. • The -COOH group is attached either to a hydrogen atom or to an alkyl group. Carboxylic acids Aliphatic Monocarboxylic Acid Aromatic Dicarboxylic Acid Polycarboxylic Acid Physical Properties • Boiling point The boiling points of carboxylic acids of similar size are higher than alcohols. The higher boiling points of the carboxylic acids are still caused by hydrogen bonding. In a pure carboxylic acid, hydrogen bonding can occur between two molecules of acid O H O C R R C O H O hydrogen bond between the fairly positive hydrogen atom and a lone pairon the fairly negative oxygen atom. There are 3 polar bonds: 1. C=O 2. C-O 3. O-H They cause reactivity high solubility in aqueous media • Carboxylic acid derivatives also contains a carboxyl group, but now there is an electronegative atom (oxygen, nitrogen, or a halogen) attached to the carboxyl carbon. This difference in structure leads to a major change in reactivity. Chemical Properties 1. Sodium Bicarbonate test (acidity test) General test for carboxylic acids O RCOH + NaHCO3 O RCO Na + H2O + CO2 bubbles Evolution of a carbon dioxide gas is a positive test for the presence of the carboxylic acid 2. Ferric Chloride test O RCOH + FeCl3 O (RCO)3Fe + 3NH3 Ammonium chloride Red Coloration Oxalic acid + Ammonium Oxalate Lime Green Benzoic acid Tan 3. Calcium Chloride test Ammonium acetate Clear Di acid + Ammonium salts White ppt