* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Somatic-derived stem cells via nuclear transfer
Survey
Document related concepts
Transcript
Lecture 2: Nuclear Reprogramming
1
Nuclear Reprogramming
Switch of gene
expression from one
cell type to another
Switch from a differentiated,
specialized cell type to a developmental
more primitive and pluripotent state
• No modification of the genome
• Alteration of the epigenome (DNA methylation, histone modification)
2
How to reprogram towards pluripotency
1.
Somatic cell nuclear transfer
1.
Somatic cell fusion with pluripotent cells
2.
Transduction of pluripotent genes into somatic cells
a.k.a. Direct reprogramming
Yamanaka and Blau, Nature, 465(7299):704{12, Jun3 2010.
History of nuclear reprogramming
nuclear transfer (blue), cell fusion (pink) and
transcription-factor transduction
green)
Yamanaka and Blau, Nature, 465(7299):704{12, Jun4 2010.
How to reprogram towards pluripotency
(reminder)
1.
Somatic cell nuclear transfer
1.
Somatic cell fusion with pluripotent cells
2.
Transduction of pluripotent genes into somatic cells
Yamanaka and Blau, Nature, 465(7299):704{12, Jun5 2010.
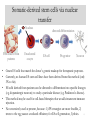
Somatic-derived stem cells via nuclear
transfer
Nucleus
Fibroblasts from
patients
Enucleated
oocyte
directed differentiation
ES cell
Progenitor
Neuron
• Create ES cells that match the donor’s genetic makeup for therapeutic purposes.
• Currently, no human ES stem cell lines have been derived from this method (only
3N so far).
• ES cells derived from patients can be directed to differentiate into specific lineages
(e.g. dopaminergic neurons) to study a particular disease (e.g. Parkinson’s disease).
• This method may be used for cell-based therapies that would circumvent immune
rejection.
• Not extensively used at present, because: 1) iPS strategies are more feasible, 2)
6
stress to the egg causes a reduced efficiency for ES cell generation, 3)ethics.
How to reprogram towards pluripotency
(reminder)
1.
Somatic cell nuclear transfer
1.
Somatic cell fusion with pluripotent cells
2.
Transduction of pluripotent genes into somatic cells
Yamanaka and Blau, Nature, 465(7299):704{12, Jun7 2010.
Cell Fusion-Mediated Nuclear Reprogramming
• Several nuclei are forced to share one common cytoplasm (viral, chemical or
electric cell fusion technologies)
• If fused cells proliferate they will become hybrids and on division the nuclei fuse to
become 4n or greater
• Ratio of different nuclei and culture medium conditions favors towards the desired
cell type.
• Direct and fast method (1-2 days)
8
How to reprogram towards pluripotency
1.
Somatic cell nuclear transfer
1.
Somatic cell fusion with pluripotent cells
2.
Transduction of pluripotent genes into somatic cells
Yamanaka and Blau, Nature, 465(7299):704{12, Jun9 2010.
Induced pluripotent stem cells (iPS cells)
10
Induced pluripotent stem cells (iPS cells)
• A type of pluripotent stem cell artificially derived from an adult
somatic cell by "forcing" expression of specific genes.
• Forced expression in somatic cells is realized by:
Skin cell
With few reprogramming factors
OCT3/4, SOX2, KLF4, cMYC
iPSC
OCT3/4, SOX2, LIN28, NANOG
Yamanaka factors
Thomson factors
– Viral transduction
– Proteins
– Plasmids
Reprogramming Strategy
– mRNA
11
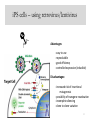
iPS cells – using retrovirus/lentivirus
Advantages:
- easy to use
- reproducible
- good efficiency
- controlled expression (inducible)
Disadvantages:
- increased risk of insertional
mutagenesis
- possibility of transgene reactivation
- incomplete silencing
- clone to clone variation
12
iPS cells – using Proteins
Advantages:
- no genomic modification
- non-DNA approach
Disadvantages:
- very slow process
- very inefficient process (0.006%, Zhou et al. 2009)
- requires the addition of other molecules (VPA)
• Reprogramming factors are fused to cell-penetrating peptide (CCP)
• Proteins can be recombinant (produced in bacteria) or in mammalian cells (HEK293)
• Proteins need to be active and functional in order to work
13
iPS cells – using Plasmids
Advantages:
- no genomic modification
Disadvantages:
- very inefficient process (0.006%, Zhou et al. 2009)
- repeated transfection
14
iPS cells – mRNA
Advantages:
- no genomic modification
- highly efficient approach
- faster kinetics
- factors titratable
- transient nature of mRNA
- Biosafety
Disadvantages:
- repeated transfection
15
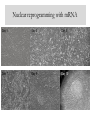
Nuclear reprogramming with mRNA
Day 1
Day 3
Day 5
Day 7
Day 9
Day 10
iPS cells - a recent advance
• it allows researchers to obtain
pluripotent stem cells, which
are important in research and
potentially have therapeutic
uses, without the controversial
use of embryos.
• Reprogramming adult cells to
obtain iPS cells may pose
significant risks that could
limit their use in humans. If
viruses are used to alter the
cells’ genome, the expression
of cancer-causing genes or
oncogenes may potentially be
triggered after these cells are
introduced into animals.
17
iPS cells versus ES cells
RNA
iPSC
Embryonic stem cell
iPS cells are believed to be similar to
ES cells with respect to:
A) stem cell gene and protein
expression
B) ability to differentiate into all
lineages in vitro
C) forming viable chimeras after
injection into blastocysts or
tumors when transplanted into
adult tissues
D) potential to form an entire
organism, such as a mouse
iPSC reprogramming factors
• Retroviruses (viruses that contain RNA, and convert RNA into DNA)
that infect fibroblast cells are commonly used.
• Virus encodes four transcription factors: Oct4, Sox2, Klf-4 and c-Myc.
C-Myc is a tumor-inducing gene (oncogene).
• Oct4 and Sox2 are necessary to induce pluripotency of fibroblasts.
• Transcription factors increase the efficiency of iPS production.
• Currently, reprogramming is inefficient and slow.
• Transcription factors modify gene expression in infected cells.
• Factors turn OFF genes that are part of the differentiated phenotype.
• Factors turn ON genes that both maintain pluripotency and the ability to
self-renew.
19
iPSC reprogramming factors – OCT3/4
• transcription factor (one slide of what a transcription
factor is!!!)
• key reprogramming factor for derivation of iPS cells
• master regulator of pluripotency
• specifically expressed in ES cells and the early embryo
• knock-down of OCT3/4 in ES cells leads to differentiation
• to date specific function of OCT3/4 during
reprogramming is not known
20
iPS reprogramming factors – SOX2
• another key factor for nuclear reprogramming
• expressed in ES cells, early embryos, germ cells and
neural stem cells
• May act as an OCT3/4 cofactor and even regulate
expression of OCT3/4 itself
• SOX2 forms heterodimers with OCT3/4 to synergistically
control ES cell-specific gene expression
21
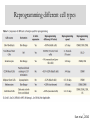
Reprogramming different cell types
22
Sun et al., 2010
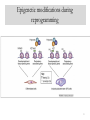
Epigenetic modifications during
reprogramming
23
What exactly happens during
reprogramming?
Suggested model #1
Suggested model #3
Suggested model #2
24
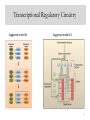
Transcriptional Regulatory Circuitry
Suggested model #1
Suggested model #2
25
Nuclear Reprogramming
Concept Mapping Terms
Add the key terms/concepts from today’s lecture to your previous
concept map. You should include (but are not limited to) the
following terms/concepts:
•Induced pluripotent stem cell
•Nuclear transfer
•Transcription factor
•Direct reprogramming
•Reprogramming factor
•Epigenome
•Transgene
•Transcriptional Regulatory Circuitry
•Yamanaka factors
Due by xxx
26