* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Fulltext - Jultika
History of invasive and interventional cardiology wikipedia , lookup
Remote ischemic conditioning wikipedia , lookup
Heart failure wikipedia , lookup
Saturated fat and cardiovascular disease wikipedia , lookup
Jatene procedure wikipedia , lookup
Cardiovascular disease wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Cardiac surgery wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Coronary artery disease wikipedia , lookup
Ventricular fibrillation wikipedia , lookup
Heart arrhythmia wikipedia , lookup
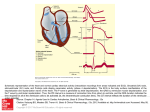
Electrocardiography wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
D 1211 OULU 2013 UNIV ER S IT Y OF OULU P. O. B R[ 00 FI-90014 UNIVERSITY OF OULU FINLAND U N I V E R S I TAT I S S E R I E S SCIENTIAE RERUM NATURALIUM Senior Assistant Jorma Arhippainen HUMANIORA University Lecturer Santeri Palviainen TECHNICA Docent Hannu Heusala ACTA U N I V E R S I T AT I S O U L U E N S I S Aapo Aro E D I T O R S Aapo Aro A B C D E F G O U L U E N S I S ACTA A C TA D 1211 ELECTROCARDIOGRAPHIC RISK MARKERS OF SUDDEN CARDIAC DEATH IN MIDDLEAGED SUBJECTS MEDICA Professor Olli Vuolteenaho SCIENTIAE RERUM SOCIALIUM University Lecturer Hannu Heikkinen SCRIPTA ACADEMICA Director Sinikka Eskelinen OECONOMICA Professor Jari Juga EDITOR IN CHIEF Professor Olli Vuolteenaho PUBLICATIONS EDITOR Publications Editor Kirsti Nurkkala ISBN 978-952-62-0178-8 (Paperback) ISBN 978-952-62-0179-5 (PDF) ISSN 0355-3221 (Print) ISSN 1796-2234 (Online) UNIVERSITY OF OULU GRADUATE SCHOOL; UNIVERSITY OF OULU, FACULTY OF MEDICINE, INSTITUTE OF CLINICAL MEDICINE; OULU UNIVERSITY HOSPITAL; HELSINKI UNIVERSITY CENTRAL HOSPITAL, HEART AND LUNG CENTER D MEDICA ACTA UNIVERSITATIS OULUENSIS D Medica 1211 AAPO ARO ELECTROCARDIOGRAPHIC RISK MARKERS OF SUDDEN CARDIAC DEATH IN MIDDLE-AGED SUBJECTS Academic dissertation to be presented with the assent of the Doctoral Training Committee of Health and Biosciences of the University of Oulu for public defence in Auditorium 4 of Meilahti Hospital, Helsinki, on 6 September 2013, at 12 noon U N I VE R S I T Y O F O U L U , O U L U 2 0 1 3 Copyright © 2013 Acta Univ. Oul. D 1211, 2013 Supervised by Professor Heikki Huikuri Docent Olli Anttonen Reviewed by Docent Antti Hedman Docent Sinikka Yli-Mäyry Opponent Docent Vesa Virtanen ISBN 978-952-62-0178-8 (Paperback) ISBN 978-952-62-0179-5 (PDF) ISSN 0355-3221 (Printed) ISSN 1796-2234 (Online) Cover Design Raimo Ahonen JUVENES PRINT TAMPERE 2013 Aro, Aapo, Electrocardiographic risk markers of sudden cardiac death in middleaged subjects. University of Oulu Graduate School; University of Oulu, Faculty of Medicine, Institute of Clinical Medicine; Oulu University Hospital; Helsinki University Central Hospital, Heart and Lung Center Acta Univ. Oul. D 1211, 2013 University of Oulu, P.O. Box 8000, FI-90014 University of Oulu, Finland Abstract Sudden cardiac death (SCD) is a major medical and public health concern responsible for 50% of cardiovascular deaths and as much as 15% to 20% of overall mortality. Coronary heart disease is the underlying cause of most of these deaths, and in 50% of such cases, SCD is the first manifestation of the disease. Researchers have investigated numerous noninvasive methods to more accurately identify individuals at high risk of SCD, but most such studies have focused on patients with specific heart disease. The standard 12-lead electrocardiogram (ECG) is a widely available tool to analyze the electrical activity of the heart, but few epidemiological studies have successfully identified specific electrocardiographic risk markers of SCD at the population level. This thesis aims to clarify the prognostic implications of several ECG patterns in the general population. We evaluated the 12-lead ECGs of 10899 middle-aged Finnish subjects (52% male) recorded between 1966 and 1972, and followed the subjects for 30 ± 11 years. The prevalence of a prolonged QRS duration ≥ 110 ms and nonspecific intraventricular conduction delay (IVCD; defined as QRS ≥ 110 ms with no partial or complete bundle branch block) in the population was 1.3% and 0.6%, respectively. Both were significantly associated with an elevated risk of all-cause mortality and cardiovascular mortality. QRS duration ≥ 110 ms doubled the risk of SCD, and IVCD was associated with a three-fold higher risk of SCD. Two percent of the subjects presented with wide frontal QRS-T angle ≥ 100° (the angle between the QRS axis and the T-wave axis in the frontal plane). A wide QRS-T angle was associated with higher overall mortality and more than doubled the risk of SCD, which was mainly due to an abnormal T-wave axis. Inverted T-waves in the right precordial leads (V1–V3) or beyond were present in 0.5% of the population. No increase in mortality or SCD was associated with right precordial T-wave inversions. In contrast, inverted T-waves in other leads than V1–V3 were associated with higher risk of cardiovascular mortality and SCD. Altogether 2.1% of the study participants presented with a prolonged PR interval > 200 ms. No rise in overall mortality, SCD, or hospitalizations due to heart failure, atrial fibrillation, or stroke was observed among these subjects during the follow-up period. In conclusion, of the electrocardiographic parameters studied, prolonged QRS duration, IVCD, and wide QRS-T angle are associated with SCD in the general population, and such changes in an ECG should therefore alert the physician to more closely evaluate and follow the patient. On the other hand, a prolonged PR interval and right precordial T-wave inversions seem to have no prognostic implications in the absence of other features suggestive of underlying heart disease. Keywords: arrhythmia, ECG, IVCD, PR interval, QRS complex, QRS-T angle, sudden cardiac death, T wave Aro, Aapo, Sydänperäistä äkkikuolemaa ennustavat tekijät EKG:ssä. Oulun yliopiston tutkijakoulu; Oulun yliopisto, Lääketieteellinen tiedekunta, Kliinisen lääketieteen laitos; Oulun yliopistollinen sairaala; Sydän- ja keuhkokeskus, HYKS Acta Univ. Oul. D 1211, 2013 Oulun yliopisto, PL 8000, 90014 Oulun yliopisto Tiivistelmä Sydänperäinen äkkikuolema on yleisin kuolinsyy länsimaissa, missä puolet sydänkuolemista ja 15–20 % kokonaiskuolleisuudesta johtuu äkillisestä sydänpysähdyksestä. Sepelvaltimotauti on yleisin taustalla oleva syy, ja jopa puolessa sepelvaltimotautikuolemista äkkikuolema on taudin ensimmäinen oire. Jo pitkään on yritetty kehittää menetelmiä, joilla voitaisiin tunnistaa suurimmassa äkkikuoleman vaarassa olevat. 12-kanavainen EKG on laajalti käytössä oleva tutkimus, jolla tutkitaan sydämen sähköistä toimintaa, mutta sydänperäistä äkkikuolemaa spesifisti väestössä ennustavia EKG-poikkeavuuksia ei ole juuri pystytty osoittamaan. Tämän väitöskirjatyön tarkoituksena oli tutkia, miten EKG:ssä nähtävät ilmiöt kuten QRS kompleksin kesto, QRS-kompleksin ja T-aallon välinen kulma, kääntyneet T-aallot sekä PR-aika korreloivat ennusteeseen väestötasolla. Tutkimme 10899 suomalaisen keski-ikäisen henkilön (52 % miehiä) EKG:t, jotka oli rekisteröity 1966–1972, ja seurasimme tutkittavia keskimäärin 30 (± 11) vuotta. Leventynyt QRS kompleksi ≥ 110 ms löytyi 1.3 %:lta ja epäspesifi kammionsisäinen johtumishäiriö eli IVCD (QRS ≥ 110 ms ilman osittaista tai täydellistä haarakatkosta) 0.6 %:lta tutkituista. Molemmat muutokset liittyivät lisääntyneeseen kokonaiskuolleisuuteen sekä sydänkuoleman riskiin. QRS kompleksin kesto ≥ 110 ms assosioitui lisäksi kaksinkertaiseen ja IVCD kolminkertaiseen äkkikuolemariskiin. 2 %:lla tutkituista sydänlihaksen depolarisaation suuntaa kuvaavan QRS-kompleksin akselin ja repolarisaatiota kuvaavan T-aallon akselin välinen frontaalitason QRS-T kulma oli leveä ≥ 100°. Näillä henkilöillä kokonaiskuolleisuus oli lisääntynyt, ja sydänperäisen äkkikuoleman riski oli yli kaksinkertainen verrattuna henkilöihin jolla QRS-T kulma oli < 100°. Oikeanpuoleisissa rintakytkennöissä V1–V3 todettiin negatiiviset T-aallot 0.5 %:lla tutkituista, mutta näillä ei ollut vaikutusta kuolleisuuteen. Sen sijaan henkilöillä, joilla todettiin negatiiviset T-aallot muissa kytkennöissä, oli yli kaksinkertainen sydänkuoleman ja äkillisen sydänpysähdyksen vaara muihin tutkittuihin verrattuna. Osallistujista 2.1 %:lla todettiin pidentynyt PR-aika > 200 ms. Tämä ei kuitenkaan vaikuttanut henkilöiden kuolleisuuteen eikä sydämen vajaatoiminnasta, eteisvärinästä tai aivoverenkiertohäiriöistä johtuvien sairaalahoitojen määrään. Tutkituista EKG:n poikkeavuuksista siis pidentynyt QRS-kompleksin kesto, IVCD ja leveä QRS-T kulma liittyvät selvästi lisääntyneeseen äkillisen sydänpysähdyksen riskiin. Sen sijaan pidentynyt PR-aika tai T-inversiot oikeanpuoleisissa rintakytkennöissä ilman muuta viitettä sydänsairaudesta eivät vaikuta ennusteeseen keski-ikäisessä väestössä. Asiasanat: EKG, IVCD, PR-intervalli, QRS kompleksi, QRS-T kulma, rytmihäiriö, sydänperäinen äkkikuolema, T-aalto To Matias and Aamos 8 Acknowledgements This study was conducted between 2009 and 2013 at the Division of Cardiology, Heart and Lung Center at the Helsinki University Central Hospital (HUCH) and at the Department of Internal Medicine at the University of Oulu. I would like to express my sincere gratitude and appreciation to Professor Heikki Huikuri for the priviledge of having him as the supervisor of this thesis. He is an internationally respected authority in the field of cardiac electrophysiology and sudden cardiac death, and his vast expertise and vision, optimism and constructive critisism have been essential to this project. I am especially thankful to him that, even though my visits to Oulu have been fewer than maybe would have been appropriate, our communication via email has always been swift, and I almost always recieved answers and comments to my questions within hours. The subject of the present thesis was proposed to me by Docent Olli Anttonen, my second supervisor. I am deeply grateful to Olli for introducing me to the magical world of research. His enthusiasm and continuous support throughout this study have proved invaluable, and I have also learned a great deal from him about clinical cardiology while I was working as a fellow in the PäijätHäme Central Hospital. My warmest thanks go to my other co-authors, whose contribution has been crucial to this project. I am deeply indebted to Jani Tikkanen for his enormous effort and efficacy in gathering data for this project and for his help in preparing the publications. I wish to thank Juhani Junttila for his innovative ideas and deep scientific knowledge that helped me maintain focus during this study project, for his valuable comments in preparing the manuscripts, and for enduring the bureaucratic task of serving as the chairperson of the follow-up committee of this thesis. Tuomas Kerola has also made an important contribution to the study and is responsible for initially persuading me to come to the Päijät-Häme Central Hospital as a fellow in cardiology, for which I am grateful. I deeply appreciate the support and positive attitude of the Institute of Health and Welfare towards this project and thankfully acknowledge the indispensible assistance of Harri Rissanen during the data analysis. I also thank Antti Reunanen for his most useful advice on the study protocol. I thank the official reviewers of this thesis, Docent Antti Hedman and Docent Sinikka Yli-Mäyry, for their valuable feedback and comments, which helped considerably to improve this thesis. 9 While carrying out my research work, I worked in clinics and specialized in cardiology, first in the Päijät-Häme Central Hospital in Lahti and later in the Helsinki University Central Hospital. In Lahti, I learned the basics of cardiology, for which I especially thank Juha Heikkilä, Liisa Kokkonen and Seppo Voutilainen. Despite the sometimes hectic pace at work, they always had the time and patience for my questions and guidance. I have recently enjoyed the priviledge and pleasure of working at the Division of Cardiology at the Helsinki University Central Hospital. I am grateful to the head of the clinic, Professor Markku Kupari, for providing an encouraging and academic athmosphere in which to work, as well as to Professor Lauri Toivonen for imparting to me some of his exceptional knowledge and perception in the field of cardiac electrophysiology. I also express my gratitude to all my colleagues for their friendship and support. I owe special thanks to Petri Korhonen, Lasse Oikarinen, Hannu Parikka, and Matti Viitasalo for sharing with me their expertise in electrophysiology, and to Sami Pakarinen and Mika Lehto for their friendly collaboration in the field of cardiac pacing. I also wish to thank Jere, Jussi, and Kristian, my roommates in the basement of the hospital, for sharing with me the joys and struggles of everyday life at work. I wish to thank my many dear friends outside this clinic, with whom I have enjoyed countless unforgettable moments, for reminding me that there is also life beyond cardiology. I am deeply grateful to my mother, Hillevi, and my late father, Seppo, for providing me with a solid foundation and unconditional love. I also wish to thank my mother and my grandparents, Raija and Pentti, and my parents-in-law, Liisa and Heimo, for their essential help in childcare and everyday life. I also thank my brother Tuomas for helping me to keep my tennis strokes in shape during these busy years. Finally, my warmest thoughts and gratitude go to my wonderful wife, Katri, for her everlasting love, patience and support, and to our children, Matias and Aamos, for bringing so much joy and happiness into our lives. The research for this thesis was supported by grants from the Finnish Foundation for Cardiovascular Research, the Finnish Medical Foundation, the Onni and Hilja Tuovinen Foundation, the Aarne Koskelo Foundation, the Aarne and Aili Turunen Foundation, and the Maud Kuistila Memorial Foundation, as well as by a special federal grant (EVO) for the Päijät-Häme Central Hospital, which I acknowledge with gratitude. 10 Abbreviations AF ARVC AV CHD CI CPVT DAD DCM EAD ECG ER HCM HF HR ICD IVCD LAHB LBBB LVEF LVH LQTS MI NYHA PEA RBBB SCD TIA VF VPB VT Atrial fibrillation Arrhythmogenic right ventricular cardiomyopathy Atrioventricular Coronary heart disease Confidence interval Catecholaminergic polymorphic ventricular tachycardia Delayed afterdepolarization Dilated cardiomyopathy Early afterdepolarization Electrocardiogram, electrocardiography Early repolarization Hypertrophic cardiomyopathy Heart failure Hazard ratio Implantable cardioverter-defibrillator Nonspecific intraventricular conduction delay Left anterior hemiblock Left bundle branch block Left ventricular ejection fraction Left ventricular hypertrophy Long QT syndrome Myocardial infarction New York Heart Association functional class Pulseless electrical activity Right bundle branch block Sudden cardiac death Transient ischemic attack Ventricular fibrillation Ventricular premature beat Ventricular tachycardia 11 12 List of original publications This thesis is based on the following publications, which are referred to in the text by their Roman numerals. I Aro AL, Anttonen O, Tikkanen JT, Junttila MJ, Kerola T, Rissanen HA, Reunanen A & Huikuri HV (2011) Intraventricular conduction delay in a standard 12-lead electrocardiogram as a predictor of mortality in the general population. Circ Arrhythm Electrophysiol 4(5): 704–710. II Aro AL, Huikuri HV, Tikkanen JT, Junttila MJ, Rissanen HA, Reunanen A & Anttonen O (2012) QRS-T angle as a predictor of sudden cardiac death in a middleaged general population. Europace 14(6): 872–876. III Aro AL, Anttonen O, Tikkanen JT, Junttila MJ, Kerola T, Rissanen HA, Reunanen A & Huikuri HV (2012) Prevalence and prognostic significance of T-wave inversions in right precordial leads of a 12-lead electrocardiogram in the middle-aged subjects. Circulation 125(21): 2572–2577. IV Aro AL, Anttonen O, Kerola T, Junttila MJ, Tikkanen JT, Rissanen HA, Reunanen A & Huikuri HV (2013) Prognostic significance of prolonged PR interval in the general population. European Heart Journal [Epub ahead of print 14 May, 2013]. 13 14 Table of contents Abstract Tiivistelmä Acknowledgements 9 Abbreviations 11 List of original publications 13 Table of contents 15 1 Introduction 17 2 Review of the literature 19 2.1 Sudden cardiac death .............................................................................. 19 2.1.1 Epidemiology and etiology........................................................... 19 2.1.2 Risk stratification ......................................................................... 21 2.2 Cardiac action potential and ion currents ................................................ 25 2.2.1 Action potential in the electrocardiogram .................................... 27 2.2.2 Mechanisms of ventricular arrhythmias ....................................... 28 2.3 Clinical significance of different electrocardiographic patterns.............. 29 2.3.1 PR interval .................................................................................... 29 2.3.2 QRS duration ................................................................................ 31 2.3.3 J wave and early repolarization .................................................... 34 2.3.4 T-wave inversion .......................................................................... 35 2.3.5 QRS-T angle ................................................................................. 37 2.3.6 QT interval ................................................................................... 39 2.3.7 Other electrocardiographic risk markers ...................................... 40 3 Purpose of the study 43 4 Participants and methods 45 4.1 Study population ..................................................................................... 45 4.2 Electrocardiographic measurements ....................................................... 45 4.3 Follow-up ................................................................................................ 47 4.4 Statistical analysis ................................................................................... 48 5 Results 49 5.1 Long-term outcome associated with prolonged QRS duration and nonspecific intraventricular conduction delay (IVCD) ........................... 49 5.2 QRS-T angle as a predictor of sudden cardiac death .............................. 51 5.3 Prevalence and prognostic significance of T wave inversions ................ 52 5.4 Prognostic significance of prolonged PR interval in middle-aged subjects .................................................................................................... 55 15 6 Discussion 59 6.1 QRS duration and IVCD as predictors of sudden cardiac death ............. 59 6.2 QRS-T angle as a risk marker of sudden cardiac death ........................... 61 6.3 Prognostic implications of right precordial T-wave inversions............... 62 6.4 Natural history of prolonged PR interval, or first-degree AV block ........................................................................................................ 64 6.5 Future aspects of electrocardiography in SCD prevention ...................... 65 6.6 Potential limitations of the studies .......................................................... 67 7 Conclusions 69 References 71 Original publications 91 16 1 Introduction Sudden cardiac death (SCD) is defined as a natural, unexpected death occurring within one hour of an acute change in clinical status. In the great majority of cases, SCD results from ventricular arrhythmias (Deo & Albert 2012). SCD accounts for an estimated 50% of all cardiovascular mortality, with coronary artery disease being responsible for up to 80% of these fatal events (Huikuri et al. 2001). Subjects with preexisting cardiac pathology and depressed left ventricular function are at elevated risk of SCD. However, at least 50% of SCD cases occur in individuals with no previously identified structural cardiac disease or in subjects considered at low risk (Zipes et al. 2006). This has lead to the investigation of a variety of noninvasive techniques in order to more accurately identify high-risk individuals before the fatal arrhythmia. Most of these studies have focused on patients with a particular cardiac disease, and apart from the traditional risk factors for atherosclerosis, epidemiological surveys have not been successful in identifying specific risk markers for SCD in the general population (Goldberger et al. 2008). The standard 12-lead electrocardiogram (ECG) has been the most important tool in clinical medicine to detect abnormalities in the electrical activity of the heart. The surface ECG records cardiac electrical fields generated by transmembrane ionic currents during the action potential of atrial and ventricular myocytes. This electrical activity of the heart was first demonstrated already in the last half of the 19th century, and in 1887, Waller first managed to record the electrical potentials of the human heart from the body surface. However, it is the recording of the human electrocardiogram by the string galvanometer, reported by Willem Einthoven in 1902, which really marks the beginning of the era of electrocardiography (Kligfield 2002). Even today, over a century after its invention, innumerable ECGs are recorded every day with the intention of diagnosing arrhythmias, myocardial ischemia, cardiomyopathies, conduction disturbances, and genetic ion channelopathies. A vast number of ECGs are also recorded from asymptomatic individuals for various reasons. Sometimes this raises the question of what the clinical significance of the electrocardiographic abnormalities incidentally found in these subjects is. For example, a prolonged PR interval, or first-degree atrioventricular block, implying decelerated atrioventricular conduction, has traditionally been considered benign (Mymin et al. 1986). In most cases, the delayed conduction occurs in the atrioventricular node, which is strongly influenced by the present 17 autonomic tone; thus, a prolonged PR interval is a frequent finding in conditioned athletes (Corrado et al. 2009). However, PR prolongation has recently been associated with elevated risk of atrial fibrillation, pacemaker implantation, and mortality (Cheng et al. 2009). Conduction delay in the His-Purkinje system can manifest as a wider QRS complex reflecting prolonged depolarization of the ventricles, usually caused by a bundle branch block. In the presence of structural cardiac disease, prolonged QRS duration has been associated with higher mortality (Desai et al. 2006, Morin et al. 2009, Schinkel et al. 2009), but in the general population, the prognostic significance of prolonged QRS duration or nonspecific intraventricular conduction delay (IVCD) is not well established. Repolarization of the ventricular myocardium manifests in the ECG mainly as the T-wave, thus abnormalities observed during the repolarization period often change the direction or shape of the T-wave. The QRS-T angle is a concept representing differences between the directions of depolarization and repolarization vectors. The spatial QRS-T angle has been studied in risk prediction in various populations, and a wide QRS-T angle has been associated with higher mortality (Kardys et al. 2003, Kors et al. 2003, Rautaharju et al. 2006b). However, an easier way to assess the QRS-T angle is from the frontal leads of the 12-lead ECG by calculating the difference between the frontal QRS axis and the T axis. Whether an abnormally wide frontal QRS-T angle has prognostic implications in the general population has not been well studied. Inverted T waves can represent another form of abnormal repolarization. In children and adolescents, T-wave inversions are common in the right precordial leads V1–V3, but these T waves become upright after pubertal development. If inverted T waves persist into adulthood, they may raise suspicions of underlying cardiac pathology, such as arrhythmogenic right ventricular cardiomyopathy (ARVC) (Marcus 2005). However, the clinical significance of inverted T waves in right precordial leads without overt cardiac disease remains unknown. The present thesis aims to study the prognostic significance of various electrocardiographic parameters (e.g. QRS duration, IVCD, PR interval, QRS-T angle, and T-wave inversion) in the general population. The ultimate goal is to be able to better identify those individuals who are at higher risk of SCD, so that preventive measures can be undertaken before this terminal event occurs. An additional purpose is to define electrocardiographic patterns which carry benign prognosis and need not worry the patient or the treating physician. 18 2 Review of the literature 2.1 Sudden cardiac death Sudden cardiac death (SCD) refers to death due to unexpected circulatory arrest, usually caused by a cardiac arrhythmia, occurring within an hour of the onset of symptoms in a person with or without previously known cardiac disease (Zipes et al. 2006). The specificity of this SCD definition varies greatly depending on whether or not the event was witnessed. A case is considered an established SCD if an unexpected death without obvious extracardiac cause is associated with witnessed collapse or if the death occurs within one hour of new or accelerating symptoms. A probable SCD, in turn, is an unexpected death with no obvious extracardiac cause, occurring in the previous 24 hours (Fishman et al. 2010). Further, the term sudden cardiac arrest describes SCD cases in which medical intervention (e.g. defibrillation) reverses the event (Zipes et al. 2006). 2.1.1 Epidemiology and etiology The annual incidence of SCD in the Western world is estimated to range from 50 to 200 per 100 000 in the general population (Fishman et al. 2010, Zipes et al. 2006). Despite great improvements in primary and secondary prevention that have substantially reduced overall coronary heart disease (CHD) mortality over recent decades, SCD rates in particular have declined to a lesser extent. Estimates indicate that SCD accounts for approximately 50% of all CHD deaths and up to 15% to 20% of all deaths (Deo & Albert 2012, Zipes et al. 2006). Ventricular fibrillation (VF), often preceded by ventricular tachycardia (VT), is the mechanism underlying most SCD episodes, but pulseless electrical activity (PEA) and bradycardias can also cease mechanical activity of the heart resulting in absence of signs of circulation (Bayes de Luna et al. 1989, Gang et al. 2010). Other potential causes of SCD include stroke, pulmonary embolism, aortic rupture, and other noncardiac causes (Estes 2011). The risk of SCD increases markedly with age, with a 100-fold lower incidence in adolescents and adults under 30 than in adults older than 35 (Zipes et al. 2006). For example, the annual incidence of SCD among 35- to 44-year-old men is around 35 per 100 000 compared with over 1300 per 100 000 for 75- to 84-year-old men (Zheng et al. 2001). The characteristics of SCD also differ 19 between men and women in several ways. Apart from the very old, women of any age have a significantly lower incidence of SCD than do men, even after adjusting for cardiovascular risk factors (Zheng et al. 2001). Approximately two-thirds of women presenting with SCD have no previously detected cardiac disease, compared with 50% in men. In addition, women suffering from SCD seem to have a higher prevalence of structurally normal hearts than do men (Chugh et al. 2009b). The pathophysiology of SCD is complex and is believed to include an abnormal myocardial or electrical substrate as well as transient factors that trigger the electrical instability and lethal ventricular arrhythmias that lead to hemodynamic collapse and cardiac arrest. However, in a significant number of cases of SCD, no apparent arrhythmia trigger can be identified (Fishman et al. 2010). The most common underlying cardiovascular condition predisposing to SCD is CHD, which accounts for approximately 80% of all SCDs. On the other hand, approximately 50% of all CHD-related deaths are due to SCD (Myerburg & Junttila 2012). The mechanisms underlying SCD in CHD include lethal arrhythmias initiated by transient ischemia, often in the absence of previously recognized coronary artery disease; arrhythmias occurring during acute myocardial infarction or in the early weeks after the acute phase during scar stabilization; and sudden cardiac arrest in the chronic phase of ischemic cardiomyopathy occurring months or years after the myocardial infarction due to ventricular remodeling and the evolution of heart failure (Myerburg & Junttila 2012). Cardiomyopathies, such as dilated cardiomyopathy (DCM), hypertrophic cardiomyopathy (HCM) and arrhythmogenic right ventricular cardiomyopathy (ARVC), account for the second largest number of sudden deaths from cardiac causes. In DCM, SCD accounts for at least 30% of overall mortality, occurring annually in 8% to 10% of New York Heart Association (NYHA) functional class I patients, and in 20% of class II–IV patients. However, at least 50% of deaths among NYHA class I DCM patients are sudden, in contrast to only 20% to 30% among NYHA class IV, in which the progressive heart failure and not the primary arrhythmia is the most common mechanism of death (Kjekshus 1990). Among patients with HCM, the combined rate of life-threatening arrhythmias and SCD is lower, around 1% per year, although the number is much higher in the presence of one or more risk factors (Sen-Chowdhry & McKenna 2012). The reported risk of SCD in ARVC varies markedly, possibly due to the different 20 baseline characteristics among patients, with annual rates of SCD or ventricular fibrillation ranging from 0.1% in affected relatives to as high as 5% to 8% in ICD recipients (Sen-Chowdhry & McKenna 2012). Other structural cardiac diseases, such as valvular and congenital heart disease, as well as acquired inflammatory and infiltrative cardiac disorders account for only a small number of SCDs occurring in the general population. Often included in the category of SCD with no overt heart disease are patients with subclinical structural cardiac diseases such as myocarditis and coronary spasm, and sometimes sarcoidosis (Modi & Krahn 2011). However, 5% to 15% of cardiac arrest victims show no evidence of structural abnormalities at autopsy (Napolitano et al. 2012). The conditions responsible for SCD in these cases are largely those that cause abnormalities in cardiac depolarization and repolarization, usually due to inherited ion channel abnormalities or metabolic-, electrolyte-, or drug-induced ion channel dysfunction. These primarily electric diseases, or channelopathies, include long-QT syndrome (LQTS), Brugada syndrome, shortQT syndrome, catecholaminergic polymorphic ventricular tachycardia (CPVT), and early repolarization syndrome (ERS). 2.1.2 Risk stratification Despite advances in cardiopulmonary resuscitation and post-resuscitation care, outcomes after cardiac arrest remain poor. Vast majority of the sudden cardiac arrests occur in the community, and 90% to 95% of these individuals do not survive, even when treated by emergency medical personnel (Nichol et al. 2008). Survival depends on the rapidity of intervention (e.g. defibrillation in VF) as well as on the presenting arrhythmia. The documented survival of patients with VF has been as high as 25%; however, when PEA presents as the initial rhythm, survival is only around 2% (Chugh et al. 2004). At least 50% of cases of SCD occur in subjects with no previously identified CHD or among subgroups of patients considered to be at low risk of SCD (Zipes et al. 2006); thus the persisting challenge has been to more accurately identify these individuals before the devastating SCD event. Since CHD accounts for as much as 80% of SCDs and pre-existing CHD itself is associated in as much as a five-fold higher risk of SCD (Cupples et al. 1992), it is logical that conventional risk factors for CHD such as age, male sex, hypertension, hypercholesterolemia, diabetes mellitus, kidney dysfunction, obesity, and smoking should predict SCD in the population (Deo & Albert 2012). Intuitively, optimization of these 21 traditional cardiovascular risk factors would lead to a lower incidence of SCD, but robust evidence supporting this view is currently lacking (Estes 2011). Left ventricular dysfunction and NYHA functional class, with either ischemic or nonischemic cardiomyopathy, are even more powerful risk factors for SCD (Stevenson et al. 1993). Reduced left ventricular ejection fraction (LVEF) is one of the strongest predictors of SCD, and in the clinical practice guidelines, LVEF < 35%, together with NYHA class, identify patients who will benefit the most from implantable cardioverter-defibrillator (ICD) therapy to prevent sudden death from arrhythmia (Zipes et al. 2006). However, even though reduced LVEF can well identify the group of patients at highest risk, the majority of SCDs will occur in the large general population of individuals without reduced LVEF and whose relative risk is considered low (Stecker et al. 2006). This problem, illustrated in Figure 1, poses a challenge to SCD-preventive strategies, since the individuals at highest risk according to current criteria comprise only a small proportion of the total number of SCD in the population. On the other hand, some patients with LVEF < 35% may still be at low risk of SCD and gain no benefit from ICD implantation (Goldenberg et al. 2008). This has generated great interest in studying different methods in order to more accurately identify individuals at high risk of SCD. 22 Fig. 1. Incidence and the annual prevalence of SCD in specific patient populations. Most SCD events occur in subjects at low to moderate risk. Modified from Myerburg et al. 1998 (published by permission of Wolters Kluwer Health). In the presence of ischemic or nonischemic heart disease, whether clinically silent or not, factors known to trigger VT or VF include changes in the autonomic nervous system, electrolyte disturbances, metabolic abnormalities, ischemia, acute volume or pressure overload of the ventricles, ion channel abnormalities, and the proarrhythmic action of cardiac or noncardiac drugs. These triggers together with underlying substrate such as scar formation, alterations in the chamber geometry, and electrical or anatomical remodeling, can induce and sustain potentially lethal ventricular arrhythmias (Goldberger et al. 2008). Detection of these arrhythmia substrates, triggering factors and abnormalities in ventricular depolarization and repolarization forms the theoretical base for the developing of different noninvasive approaches with the purpose of enhancing SCD risk stratification. To identify imbalances in autonomic function, studies have used techniques such as heart rate variability, baroreflex sensitivity, heart rate turbulence, and heart rate recovery after exercise to stratify risk of SCD among cardiac patients (Goldberger et al. 2008). The extent of myocardial damage and LVEF have traditionally been assessed with echocardiography, but recent studies have 23 suggested that contrast enhanced magnetic resonance imaging can be of additional value in detecting the arrhythmia substrate by carefully delineating the extent and morphology of myocardial scar tissue (Villuendas & Kadish 2008). Prolonged duration of ventricular depolarization in an electrocardiogram (ECG) due to intraventricular or interventricular conduction delay or block may reflect the presence of myocardial damage and scar tissue, and can manifest as increased duration of the QRS complex. Signal-averaged ECG can serve to detect late activation within the myocardium, known as late potentials, that occur after the end of the QRS complex and are so small in amplitude that several hundred QRS complexes are needed to average for their detection. Prolonged duration of the filtered QRS, low-amplitude signal duration and abnormally low voltage of the terminal 40 ms of the QRS are the parameters commonly assessed for evidence of late potentials, which can correspond to the substrate of sustained ventricular arrhythmias (Stein 2008). Heterogeneities in ventricular repolarization can also predispose individuals to risk of SCD. Besides the QT interval, QT interval variability and T-wave alternans have been employed to detect repolarization abnormalities (Goldberger et al. 2008). Finally, the presence of ventricular premature beats (VPBs) and nonsustained ventricular tachycardia could trigger a fatal arrhythmia, so several studies have evaluated their value in risk stratification (Stein 2008). In summary, specific efforts to prevent SCD have focused on the population groups at greatest risk (e.g. patients with previous malignant ventricular arrhythmias, severely depressed left ventricular function due to ischemic or nonischemic cardiomyopathy, and symptomatic primary electrical diseases). In these patients, treatment of ventricular arrhythmias with an implantable cardioverter-defibrillator (ICD) has led to improved survival rates in several large clinical trials (Epstein et al. 2008). However, because the subjects at highest risk account for only a minority of SCD cases, preventing SCD in this population will only slightly reduce the total number of SCD victims. Consequently, researchers are searching for other specific risk markers with the aim of identifying high-risk individuals in the general population. Indeed, many noninvasive techniques have been developed and studied with the intention to more accurately identify individuals at elevated risk of fatal arrhythmias. However, the ability of each of these techniques to provide additional prognostic information varies, so the best way to combine and use these techniques in clinical practice remains unclear. Low LVEF < 35% is the parameter most widely used to decide whether to implant an ICD for the primary prevention of SCD, but how to optimally define 24 the group of patients who do not have low LVEF but are nonetheless at substantial risk of SCD remains to be determined (Goldberger et al. 2008). Many of the methods described above also require special equipment or software and have limited use outside clinical trials. In contrast, the standard 12-lead electrocardiogram is readily available, and its role in detecting abnormalities in ventricular depolarization and repolarization and risk stratification has recently attracted growing interest. 2.2 Cardiac action potential and ion currents Contraction of the cardiac muscle cells, cardiomyocytes, is initiated by action potentials. Disorders of this electrical activity can create serious and potentially lethal disturbances in cardiac rhythm. Action potentials occurring in normal atrial and ventricular myocytes are divided into five phases. During the resting phase, the interior of the cell has more negative voltage than the exterior, with a membrane voltage of around –90 mV. This gradient results from different intracellular and extracellular ion concentrations that various ion transporters and pumps maintain with energy-dependent mechanisms. The stimulation of excitable myocytes evokes depolarization, which suddenly renders the membrane potential of the cells positive. This change is then followed by a gradual repolarization of the membrane voltage until restoration of the original negative resting value. This action potential is rapidly conducted throughout the heart and is responsible for initiating each heartbeat (Rubart & Zipes 2008). The different phases of the cardiac action potential are associated with changes in cell membrane permeability, mainly to Na+, K+, Ca++ and Cl- ions, thus inducing changes in the membrane voltage (Pappano 2008). 25 Fig. 2. Different phases of the ventricular action potential and some of the underlying ionic currents. Modified from Nerbonne & Kass 2005 (published by permission of the American Physiological Society). Different phases of the action potential and the principle ion currents responsible for these changes in cardiac myocytes appear in Figure 2. Rapid depolarization or upstroke is initiated in phase 0, when a depolarizing stimulus exceeds a certain threshold potential. This induces a dramatic change in the cell membrane properties, causing fast Na+ channels to rapidly open; Na+ thus enters and thereby depolarizes the myocyte. These Na+ channels then abruptly inactivate and remain so until the cell becomes fully repolarized. Repolarization begins in phase 1 with a brief period of activation of a transient outward current (Ito) carried mainly by K+, which creates the notch in the action potential between the end of the upstroke and the beginning of the plateau (Pappano 2008). The plateau (phase 2) may last several hundred milliseconds and is mainly maintained by a balance between outward current carried by K+ moving 26 through IK, IK1, and Ito channels, and inward current carried by Ca++ through open L-type Ca++ channels (ICa.L), which serves as a trigger for the cardiac muscle contraction (Pappano 2008). Final rapid repolarization (phase 3) begins when the efflux of K+ ions from the cells begins to exceed the influx of Ca++. The transient outward (Ito) current together with the slow and rapid components of the delayed rectifier potassium current (IKs and IKr) together initiate the final repolarization; once phase 3 has been initiated, the inwardly rectified current (IK1) influences the rapidity of the repolarization (Pappano 2008). In phase 4, the membrane potential has returned to the resting voltage and remains steady throughout diastole in atrial and ventricular cells, which is mainly the contribution of the efflux of K+ by inwardly rectifying K+ channels (IK1) compensating for the slow influx of the Na+ ions. However, in the sinus node, certain parts of the atria, the distal portion of the atrioventricular (AV) node, and the His-Purkinje fibers, the resting membrane potential does not remain constant but gradually depolarizes, and in the absence of a stimulus to generate rapid depolarization, these cells can themselves initiate a spontaneous action potential (Rubart & Zipes 2008). 2.2.1 Action potential in the electrocardiogram A normal cardiac cycle comprises two basic electrocardiographic processes: depolarization and repolarization of the atria and ventricles. Under normal conditions, the sinus node is the pacemaker of the heart and initiates the spread of action potentials throughout the atria. This atrial depolarization represents the P wave in the ECG. Atrial repolarization is normally of low amplitude and obscured by the QRS complex, so it is seldom visible in the ECG. Atrial action potential reaches the ventricles via the AV node, where the conduction is significantly delayed in order to permit optimal ventricular filling during atrial contraction. On the ventricular side, the excitation wave passes to the bundle of His, which then divides into the right and left bundle branches. The left bundle branch splits into a thin anterior division and a thick posterior division, and ultimately the right bundle branch and the two divisions of the left bundle branch subdivide into a complex network of Purkinje fibers (Pappano 2008). The propagation of ventricular depolarization manifests in the electrocardiogram as the QRS complex, with its morphology and electrical axis depending on the endocardial and transmural activation sequences of the ventricles. The rapid phase of the repolarization of the ventricles is mainly 27 depicted in the ECG as the T wave, but the ST segment, the J-wave and sometimes the U wave are also considered to represent the repolarization (Yan et al. 2003). Although activation of the ventricles moves from the endocardium to the epicardium, the ventricular electrical recovery, in contrast to ventricular activation, passes from the epicardium to the endocardium. As a result, the QRS complex, the ST segment and T waves are generally concordant (i.e. pointing to the same direction in healthy individuals) (Mirvis & Goldberger 2008). 2.2.2 Mechanisms of ventricular arrhythmias Potential mechanisms of ventricular arrhythmias include automaticity, reentry, and triggered activity due to early or delayed afterdepolarizations. In addition, the heterogeneity of local action potential durations facilitates the genesis of certain arrhythmias (Josephson 2008b). An episode of arrhythmia caused by one mechanism can also precipitate an arrhythmia caused by a different mechanism, as when a premature ventricular complex caused by automaticity initiates an episode of ventricular tachycardia sustained by reentry. Automaticity refers to the property of cardiac cells to initiate action potential spontaneously without the need for prior stimulation. In contrast, triggered activity occurs as a result of a preceding impulse. In the ventricles, these afterdepolarizations have been demonstrated in the Purkinje fibers and the ventricular myocardium (Rubart & Zipes 2008). Early afterdepolarizations (EADs) can occur before full repolarization of the cardiac cells in phases 2 or 3 of the action potential and are related to increased Ca2+ influx through L-type Ca2+ channels. They are more likely to occur in cardiac cells with prolonged action potential at slower heart rates. EAD are thought to be responsible for torsades de pointes (TdP) associated with long-QT syndrome and some antiarrhythmic drugs that prolong the cardiac action potential (Rubart & Zipes 2008). Delayed afterdepolarizations (DADs) occur after the completion of repolarization (phase 4) and are associated with elevated intracellular calcium concentrations. DADs are more likely to occur with higher heart rates and have been linked to arrhythmias in, for example, catecholaminergic polymorphic ventricular tachycardia (CPVT) (Rubart & Zipes 2008). In patients with prior myocardial infarct, reentry is the predominant mechanism of ventricular tachycardia. Necessary conditions for reentry are a closed conduction loop, an unidirectional block at some point of the reentry circuit, and an area of sufficiently slow 28 conduction enabling continuous propagation of the impulse (Rubart & Zipes 2008). 2.3 Clinical significance of different electrocardiographic patterns Numerous studies, conducted among subjects both with and without structural heart disease, have addressed the prognostic significance of several electrocardiographic patterns. Especially in the general population, the positive predictive value and specificity of these risk markers have remained relatively low. Despite this, research has successfully identified several changes in the ECG that have been associated with higher risk of cardiovascular mortality and morbidity. 2.3.1 PR interval The time from the beginning of atrial depolarization to the onset of ventricular depolarization measured for the surface ECG is known as the PR interval. The upper limit of the PR interval following a normally timed P wave is 200 ms, and prolongation of the PR interval > 200 ms is known as a first-degree atrioventricular (AV) block. A second-degree AV block is defined as the inconsistent conduction of atrial impulses to the ventricles, and in a third-degree AV block, no atrioventricular conduction occurs. A prolonged PR interval can result from a conduction delay in the atrium (intra-atrial delay), the AV node, and the His-Purkinje system. In most cases, the AV node is responsible for the delayed conduction, although more than one site can contribute to a prolonged PR interval in an individual patient (Schwartzman 2004). The PR interval and heart rate share an inverse relationship; the PR interval becomes shorter with higher rates and vice versa (Atterhog & Loogna 1977). The autonomic nervous system strongly influences impulse propagation in the AV node (Josephson 2008a); consequently, the PR interval also shows significant circadian variation depending on the prevailing vagal tone (Dilaveris et al. 2001). Among trained athletes with higher parasympathetic tone and lower resting sympathetic activity, a prolonged PR interval mediated by delayed conduction in the AV node may occur in > 10% of the individuals (Corrado et al. 2009). Evidence also suggests that genes contribute considerably to the cardiac conduction. The PR interval reportedly has significant hereditability (NewtonCheh et al. 2007, Smith et al. 2009), and several common genetic variants seem 29 to modulate heart rate, PR interval, and QRS duration (Holm et al. 2010, Pfeufer et al. 2010). This is in accordance with a recent report that showed prolonged PR interval and other conduction abnormalities to be highly prevalent among the parents of children with idiopathic congenital AV block (Baruteau et al. 2012). In the general population, the prevalence of prolonged PR interval is around 0.5% to 1.5% in young adults from 20 to 40 years of age, 2% to 3% in subjects between 40 and 60 years of age, and still higher with advanced age (Bexton & Camm 1984, Hiss & Lamb 1962, Mymin et al. 1986, Perlman et al. 1971, Rose et al. 1978). Traditionally, decelerated AV conduction has been considered a benign, functional phenomenon. Most early studies of the prognostic significance of prolonged PR duration examined only young and healthy men from the military. The first estimation of the prognosis associated with the PR interval involved 1000 young aviators from the US Army, but during the ten-year follow-up period, heart disease proved very rare and only one cardiac death occurred, rendering impossible the detection of any differences in outcomes in the few subjects with prolonged PR interval (Packard et al. 1954). Further studies conducted with nearly 4000 pilots recruited after World War II with a 30-year follow-up period (Mymin et al. 1986), and with 18 000 middle-aged male civil servants with a 5year follow-up (Rose et al. 1978), demonstrated no differences in the prognosis of subjects with prolonged PR interval compared to that of the rest of the population. However, one international study of over 12 000 men suggested that prolonged PR interval might be associated with coronary artery disease during the five-year follow-up, but the analysis was unadjusted and the number of events was low (Blackburn et al. 1970). On the other hand, another study reported that prolonged PR interval was associated with fewer coronary events in their study sample (Erikssen & Otterstad 1984). Only two major community-based studies have investigated the prognostic significance of prolonged PR interval in the general population. The first, conducted among 4678 participants in the 1960s in Tecumseh, Michigan, with a follow-up of four years, demonstrated no significant difference in cardiovascular morbidity or mortality between subjects with PR ≥ 220 ms compared to the rest of the population; among those over 60 years of age, however, there seemed to be a correlation with cardiac disease (Perlman et al. 1971). Thus, until recently, a prolonged PR interval, or first-degree AV block, has been considered a benign finding in the ECG. However, a recent report from the Framingham Heart Study population has challenged this longstanding perception. In their general population-based sample of 7575 individuals, PR interval > 200 ms predicted 30 overall mortality with a multivariable-adjusted hazard ratio of 1.4 during the follow-up period that was censored after 20 years. The participants with a prolonged PR interval experienced significantly more atrial fibrillation, and pacemaker implantations were also more common in this group than in those with normal PR interval (Cheng et al. 2009). 2.3.2 QRS duration The QRS complex on the electrocardiogram marks the rapid depolarization of the ventricular myocardium. Abnormalities in the intraventricular propagation of supraventricular impulses prolong QRS duration and can alter the shape of the QRS complex. These changes in ventricular depolarization may result from abnormalities in the His-Purkinje conduction system or the ventricular myocardium due to fibrosis, necrosis, calcification, infiltrative lesions, or ischemia, or they can be functional and depend on the current heart rate. In addition, abnormal atrioventricular connections can produce QRS widening due to ventricular preexcitation. According to the AHA/ACC/HRS recommendations for the standardization and interpretation of the electrocardiogram on intraventricular conduction disturbances published in 2009, QRS duration over 110 ms is considered abnormal in adults (Surawicz et al. 2009). Electrocardiographically, a prolonged QRS may result from incomplete or complete left and right bundle branch block (LBBB and RBBB), as well as nonspecific intraventricular conduction disturbance (IVCD). In complete bundle branch block, QRS duration is ≥120 ms. In incomplete LBBB and RBBB, QRS duration falls between 110–119 ms, but the morphology of the QRS complex is otherwise similar to that of a complete bundle branch block. According to these criteria, IVCD is defined as QRS duration greater that 110 ms but not fulfilling the criteria for incomplete or complete LBBB or RBBB (Surawicz et al. 2009). A summary of the diagnostic criteria for complete bundle branch blocks appears in Table 1. 31 Table 1. Diagnostic criteria of complete LBBB and RBBB according to Surawicz et al. (2009). LBBB RBBB QRS ≥ 120ms QRS ≥ 120ms Broad R waves in I, aVL, V5, and V6 rsr’, rsR’, or rSR’ in V1 or V2 (occasionally RS pattern in V5 and V6) (R’ or r’ usually wider than the initial R wave) R peak time > 60 ms in V5 and V6, but normal in V1–V3 S wave duration greater than R wave or > 40 ms (when small initial r waves present) in I and V6 ST and T waves usually opposite to QRS direction Normal R peak time in V5 and V6, but > 50 ms in (positive T wave in leads with upright QRS may be V1 (when pure dominant R wave with or without normal) a notch in V1) Q waves absent in I, V5, and V6 Prolonged duration of the QRS complex has generally been associated with adverse prognosis in patients with cardiac disease. In patients hospitalized for heart failure, LBBB is the most common cause of prolonged QRS duration, followed by IVCD and RBBB, respectively (Sandhu & Bahler 2004). In these patients, QRS duration is associated with more advanced left ventricular dysfunction and is also an independent predictor of morbidity and mortality (Kashani & Barold 2005, Wang et al. 2008). In the presence of coronary artery disease, prolonged QRS duration is associated with increased risk of sudden death (Teodorescu et al. 2011), and in patients with acute coronary syndrome, QRS prolongation, particularly in the presence of LBBB, associates with additional mortality (Baslaib et al. 2010). When patients with coronary artery disease also have depressed left ventricular function, QRS prolongation resulting from LBBB or IVCD predicted a 50% increase in their risk of arrhythmic death and total mortality (Zimetbaum et al. 2004), and even when coronary artery disease is only suspected, QRS duration is an independent predictor of cardiac death and nonfatal myocardial infarction (Schinkel et al. 2009). In a general medical population sample without bundle branch block, those with QRS duration > 130 ms were at nearly twice the risk of cardiovascular death as those with QRS duration ≤ 110 ms, and in this population the presence of LBBB and RBBB together with markedly prolonged QRS duration associated with a greater risk of cardiovascular death (Desai et al. 2006). In addition, subjects with hypertrophic cardiomyopathy (Bongioanni et al. 2007) and asymptomatic patients with aortic stenosis (Greve et al. 2012) presenting with prolonged QRS duration are at greater risk of cardiovascular and sudden cardiac death. In hypertensive patients with electrocardiographic left ventricular 32 hypertrophy (LVH), QRS duration predicts cardiovascular and all-cause mortality (Morin et al. 2009, Oikarinen et al. 2004) and identifies patients at higher risk of SCD (Morin et al. 2009). Most studies of QRS duration conducted in the general population have examined only the prognostic significance of prolonged QRS duration attributable to RBBB and LBBB. While several studies among patients with bundle branch block and concomitant cardiovascular disease have indicated higher mortality (Freedman et al. 1987, Go et al. 1998, Newby et al. 1996, Sumner et al. 2009), studies on the prognostic significance of RBBB and LBBB in the general population have yielded conflicting results, partly due to small sample sizes, short follow-up periods, or different characteristics of the subjects. Complete RBBB is an exceptionally rare finding in young adults, but its prevalence increases rapidly with advancing age (Bussink et al. 2013, Thrainsdottir et al. 1993). Complete RBBB may be associated with various cardiac conditions such as ischemic heart disease, different cardiomyopathies, myocarditis, hypertension, congenital heart disease, cor pulmonale, and pulmonary embolism (Fernández-Lozano & Brugada 2013), but in the absence of any overt cardiac disease, RBBB is generally not associated with higher mortality risk, although it can predict a future high-degree atrioventricular block (Eriksson et al. 2005, Fahy et al. 1996, Schneider et al. 1981). However, a recent report from the Copenhagen City Heart Study reported higher cardiovascular mortality associated with RBBB. In the study, age, male gender, and higher blood pressure associated with incident RBBB (Bussink et al. 2013). Similarly to RBBB, LBBB seldom occurs in subjects under the age of 55, but its incidence grows significantly with age (Haataja et al. 2012). In many cases, LBBB may be the first manifestation of underlying cardiac disease. However, abnormal asynchronous contraction patterns induced by LBBB can compromise ventricular performance even in the absence of identifiable structural cardiac disease, and the adverse impact of LBBB on cardiac function can often be reversed by the resynchronization of systole with biventricular pacing. Besides dyssynchrony and reduced left ventricular function, LBBB can induce regional ischemia, cause an abnormal increase in pulmonary artery pressure during exercise, and provoke functional mitral regurgitation (Breithardt & Breithardt 2012). It is therefore unsurprising, that compared not only with normal intraventricular conduction, but also with RBBB, LBBB has generally been associated with poorer prognosis, with a number of studies suggesting that LBBB 33 is associated with greater risk of cardiovascular mortality and morbidity (Eriksson et al. 2005, Fahy et al. 1996, Miller et al. 2005, Rabkin et al. 1980). Only a few studies have investigated the prognostic significance of prolonged QRS duration and IVCD in the general population. In a community-based cohort of the Framingham Heart Study, even minor QRS prolongation indicated a greater risk of pacemaker implantation, even after excluding subjects with bundle branch block (Cheng et al. 2010). More recently, Kurl et al. (2012) demonstrated that among middle-aged men from Eastern Finland, each 10 ms increase in QRS duration associated with a 27% higher risk of SCD. In that study, the subjects with QRS over 110 ms were at a 2.5-fold higher risk of SCD than were those with QRS duration in the lowest quintile, and the results remained similar even after excluding men with bundle branch block. 2.3.3 J wave and early repolarization The J wave is the deflection sometimes observed following the QRS complex on the surface electrocardiogram. An early repolarization (ER) pattern is a broad expression referring to distinct J waves or J-point (QRS-ST junction) elevation, notching or slurring of the terminal QRS complex, and ST segment elevation (Antzelevitch et al. 2011). For decades, the pattern of J-point elevation predominantly found in the mid-precordial leads V2 to V4 of healthy young men has been considered a benign phenomenon in the absence of symptoms attributable to myocardial ischemia or pericarditis (Klatsky et al. 2003, Rautaharju et al. 2009, Uberoi et al. 2011). The J wave, previously also known as the Osborn wave, can occur in various cardiac and extracardiac conditions, such as hypothermia, hypercalcemia, hypervagotonia and brain injury (Derval et al. 2011). Recently, however, this Jwave pattern, especially in the inferior and lateral leads accompanied by slurring or notching of the terminal QRS complex, has been associated with vulnerability to ventricular fibrillation (Haissaguerre et al. 2008, Nam et al. 2008). The precise etiology of the J wave has yet to be determined, but it likely results from transmural differences in the early phases of cardiac action potential (Antzelevitch et al. 2011). Both ER syndrome, which is associated with lifethreatening arrhythmias, and Brugada syndrome present somewhat similar abnormal J-wave manifestations, although in different regions of the heart; consequently, some authors have proposed the expression “J wave syndrome” to describe these phenotypic changes (Antzelevitch et al. 2011). 34 The ER pattern occurs in 1% to 9% of the general population, but it is found in 15% to 70% of cases of idiopathic ventricular fibrillation (VF) (Benito et al. 2010). In the Finnish population, ER in the inferior leads with a J-point elevation of more than 0.2 mV was associated with greater risk of cardiac and sudden arrhythmic death (Tikkanen et al. 2009). In athletes, ER is a very frequent finding, but is mainly associated with an ascending ST segment, which is not associated with higher risk of arrhythmic death. In contrast, when a horizontal or descending ST segment accompanies ER, the risk of arrhythmic death is higher, especially in the presence of a higher amplitude ER pattern (Tikkanen et al. 2011). The several different J-wave or ER patterns carry different prognoses. The inferolateral ER pattern associated with idiopathic VF seems to be an entity resembling Brugada syndrome. In contrast, the inferolateral ER pattern associated with greater mortality risk in the general population may more closely resemble a risk modifier that exposes an individual to an increased risk of proarrhythmia in the presence of other risk factors, such as ischemia (Junttila et al. 2012). 2.3.4 T-wave inversion The T wave corresponds to the phase of rapid repolarization (phase 3) of the ventricular action potential. Polarity of the T wave is normally concordant with the QRS complex, suggesting that normal ventricular electrical recovery, unlike ventricular activation, spreads from the epicardium to the endocardium (Burgess 1979). After the discovery of midmyocardial M cells, it is now generally accepted that the ventricular myocardium is not uniform, but composed of at least three cell types with distinct electrophysiological properties, with M cells having longer action potential duration than the endocardial and epicardial cells (Yan et al. 1998). According to this theory, the genesis of an upright T wave begins during the plateau phase with separation of the epicardial action potential from that of the M cells stemming from earlier repolarization of the epicardial cells. The peak of the T wave coincides with the full repolarization of the epicardium, and the gradual repolarization of the endocardium and M cells contributes to the descending limb of the T wave, which ends when the M cells are fully repolarized (Yan et al. 2003). In the 12-lead ECG of adults, the normal T wave is inverted in aVR; upright or inverted in leads aVL, III, and V1; and upright in limb leads I and II, as well as in precordial leads V3 to V6. In general, the T-wave amplitude is most positive in leads V2 and V3. Minor T-wave inversions in V2 and aVF can exhibit normal 35 variation, especially in young adults (Rautaharju et al. 2009), and is not considered a codeable abnormality according to the Minnesota code classification system for electrocardiographic findings (Prineas et al. 1982). Several benign and pathological conditions can induce changes in repolarization detected as an alteration of the ST segment or T wave. These primary repolarization abnormalities include myocardial ischemia and other structural heart diseases, electrolyte disturbances, drugs, changes in sympathetic tone, and hyperventilation (Alexopoulos et al. 1996, Atterhog et al. 1981, Blom 1951, Rautaharju et al. 2009, Toivonen et al. 1997). Abnormal ST segment and Twave patterns can also stem from changes in the sequence and duration of ventricular depolarization attributable to bundle branch blocks, ventricular preexcitation, and ventricular pacing, that cause secondary changes in repolarization (Rautaharju et al. 2009). Changes in the ST segment and T waves can be early markers of underlying cardiovascular disease, and even minor ST-T abnormalities have predicted reduced survival rates in the adult population (Cuddy & Tate 2006, Greenland et al. 2003b, Sigurdsson et al. 1996). However, the clinical significance of inverted T waves in the right precordial leads V1–V3 is less clear. In children and adolescents, T-wave inversion is a common physiological finding in chest leads beyond V1, but these juvenile T-wave inversions usually become upright after pubertal development (Suarez & Suarez 1946). In 0.1% to 3% of apparently healthy individuals, however, this electrocardiographic pattern persists into adulthood and most commonly occurs in young women (Hiss et al. 1960, Marcus 2005, Papadakis et al. 2009, Pelliccia et al. 2007). Although right precordial T-wave inversions can be just an electrocardiographic variation of no clinical significance, it can also be a sign of underlying structural heart disease such as hypertrophic cardiomyopathy, myocardial ischemia, or ARVC (Okada et al. 1994, Thiene et al. 1988). In a report by Pellicia et al. (2008) from Italy, the prevalence of marked T-wave inversions was 1% in young, trained athletes, over one-third of whom demonstrated evidence of structural heart disease. Disturbingly, in spite of thorough initial workup to diagnose underlying cardiac diseases in these patients, 6% of the individuals with inverted T-waves but no initial evidence of cardiac abnormalities ultimately proved to have cardiomyopathy during the follow-up. However, in another Italian study of asymptomatic children with inverted Twaves at preparticipation screening, only 2.5% were diagnosed with cardiomyopathy (Migliore et al. 2012). 36 Abnormalities of repolarization observed in a standard 12-lead ECG could imply the presence of a structural heart disease such as hypertrophic cardiomyopathy or coronary artery disease, but they play an especially important role in the diagnosis of ARVC. ARVC is a predominantly genetically determined myocardial disease characterized pathologically by replacement of the right ventricular myocardium with adipose and fibrous tissue. Clinical manifestations of the disease include ventricular arrhythmias, heart failure and SCD (Basso et al. 2009, Marcus et al. 1982). The diagnosis of ARVC is based on the presence of abnormalities in several categories, including structural and histological alterations of the right ventricle, depolarization and repolarization abnormalities in the ECG, arrhythmias, and family history. Recently, the old diagnostic criteria for diagnosing ARVC were modified in order to facilitate diagnosis (Cox et al. 2010, Marcus et al. 2010, McKenna et al. 1994). In the category of depolarization abnormalities, an epsilon wave in the right precordial leads is considered a major criterion. The prevalence of an epsilon wave ranges from 8% to 33% in ARVC patients (Cox et al. 2009, Nasir et al. 2004), but an epsilon wave in the general population is a very rare finding (Uhm et al. 2011). Repolarization abnormalities seem to be early and more sensitive markers of disease expression in ARVC, and inverted T waves through V3 have demonstrated the most optimal sensitivity and specificity for identifying these patients (Jain et al. 2009). The prevalence of right precordial T-wave inversions in ARVC is reportedly as high as 85% (Nasir et al. 2004), with the extent of inverted T waves reflecting the degree of right ventricular involvement (Nava et al. 1988). Therefore, in the repolarization category of the new guidelines for the diagnosis of ARVC, T-wave inversions in the right precordial leads V1–V3 were upgraded as major, and T-wave inversion in V1–V2 or V4–V6 as minor criteria. In the presence of complete RBBB, inverted T waves beyond V3 are considered minor criteria (Marcus et al. 2010). 2.3.5 QRS-T angle Abnormalities of ventricular depolarization depicted in the QRS complex reflect changes in impulse propagation through the conduction system and ventricular myocardium, whereas repolarization abnormalities observed as changes in the ST segment and T wave represent regional pathophysiological changes in the ionic channels that may be associated with electrical instability. Combining both 37 phenomena may thus provide addition information on the heterogeneities of ventricular action potential. The spatial QRS-T angle corresponds to the angle between the QRS complex and the T wave in three-dimensional space. A similar measurement sometimes used to define the global angel between depolarization and repolarization is total cosine R-to-T (TCRT). The spatial QRS-T angle has been demonstrated to predict outcomes in different patient populations (Kors et al. 2003, de Torbal et al. 2004, Rautaharju et al. 2007, Zabel et al. 2000). In the Women’s Health Initiative study, wide QRS-T angle was a significant predictor of congestive heart failure, coronary heart disease events, and mortality in postmenopausal women (Rautaharju et al. 2006a, Rautaharju et al. 2006b); also, in the clinical setting, the spatial QRS-T angle has proved to be a significant and independent predictor of cardiovascular mortality (Yamazaki et al. 2005). Moreover, in a general population cohort of 6134 women and men aged 55 years and older from the Rotterdam Study, Netherlands, abnormal spatial QRS-T angle was strongly associated with cardiac and sudden arrhythmic death, as well as all-cause mortality (Kardys et al. 2003). Although information about the spatial QRS-T angle could be incorporated into the automated reports of modern electrocardiographic machines, it is generally unavailable in clinical practice (Okin 2006). However, frontal QRS and T-wave axes are generally included in ECG reports and can also be fairly easily estimated from the limb leads. It is thus possible to simply calculate the frontal QRS-T angle as the difference between the frontal plane QRS axis and the T axis. The normal limits of the frontal QRS-T angle vary according to age and sex, with the difference between the lower and upper normal values ranging from around 100° to 120°. For example, the normal limits of the QRS-T angle are between 47° to 61° in women aged 30 to 39, and between -89° and 26° in women over 50 years of age (Macfarlane & Lawrie 1989). The frontal QRS-T angle can sometimes underestimate the true spatial QRS-T angle (Macfarlane 2012), but it has proved to be a suitable clinical substitute for the spatial QRS-T angle for risk prediction, as reported in the ARIC Study (Zhang et al. 2007). Frontal QRS-T angle > 90° has shown additional prognostic value in patients with nonischemic cardiomyopathy (Pavri et al. 2008) and can predict ventricular arrhythmias in patients with ischemic heart disease (Borleffs et al. 2009). An abnormal frontal QRS-T angle can result from an abnormal QRS-axis or T-wave axis. Normally, frontal QRS-axis lies between -30° and +90°, and the Twave axis between 0° and 75°. A rightward QRS axis is rare and can result from, 38 for example, right ventricular hypertrophy and pulmonary diseases (Mirvis & Goldberger 2008), whereas a left-axis deviation occurs more frequently. Potential causes for leftward deviation of the QRS axis include left ventricular hypertrophy, horizontal position of the heart in the chest, and inferolateral myocardial infarction, but it is most commonly due to left anterior hemiblock (LAHB), which is generally associated with a benign prognosis (Elizari et al. 2007). In contrast, a shift in the T-wave axis reportedly indicates mortality risk (de Torbal et al. 2004). In an elderly population with no overt coronary heart disease, Rautaharju et al. (2001) reported that a spatial T axis was associated with increased risk of incident coronary heart disease events, cardiovascular deaths, and all-cause mortality. Kors et al. (1998) obtained similar results from the Rotterdam study, in which participants with an abnormal T axis had substantially higher risk of cardiac mortality and SCD. 2.3.6 QT interval The QT interval is measured from the onset of the QRS complex to the end of the T wave and represents the sum of the ventricular action potential durations. The QT interval shortens as the heart rate increases and is commonly corrected (QTc) with Bazett’s formula (the QT interval divided by the square root of the R-R interval), although widely acknowledged limitations of this method have led to the proposition of various other approaches for QT-rate correction (Rautaharju et al. 2009). The QT interval is slightly longer in women, but this gender difference becomes less prominent in older age groups. Some discussion has focused on the appropriate upper normal limits of the QT interval, but recent recommendations consider QTc 450 ms or more in men and QTc 460 ms or more in women to be prolonged (Rautaharju et al. 2009). Several other factors (e.g. hypokalemia, hypomagnesemia, hypocalcemia, myocardial ischemia, cardiomyopathies, autonomic influences, and hypothermia) also influence the QT interval, but the most important clinical causes of prolonged QT interval are numerous QTprolonging drugs and long QT syndrome (LQTS) (Goldenberg & Moss 2008). LQTS is an arrhythmogenic disorder in a structurally normal heart presenting with a prolonged QT interval associated with peculiar T-wave morphology, syncope, and SCD. In most cases, LQTS results from a decrease in repolarizing K+ currents or an inappropriately late entry of Na+ into the myocyte (Goldenberg & Moss 2008) with an estimated prevalence of approximately 1:2000 (Schwartz et al. 2009). However, QTc prolongation has also been associated with increased 39 risk of SCD in non-LQTS population-based cohorts (Algra et al. 1991, Straus et al. 2006, Zhang et al. 2011) as well as in the presence of coronary artery disease (Chugh et al. 2009a), generating interest in the potential role of prolonged QTc in the SCD risk prediction. In contrast, short QTc intervals show no association with adverse outcomes in the general population (Anttonen et al. 2007), although short QTc intervals occur in short QT-syndrome, which is associated with hastened ventricular repolarization and higher risk of SCD (Giustetto et al. 2006). 2.3.7 Other electrocardiographic risk markers Left ventricular hypertrophy Left ventricular hypertrophy (LVH) is an indicator of target organ damage in chronic hypertension and in various studies has predicted greater morbidity and mortality (Hsieh et al. 2005, Sullivan et al. 1993, Tikkanen et al. 2009). The prevalence and prognostic significance of electrocardiographic LVH varies depending on the criteria used (Hancock et al. 2009), and in the presence of LVH, P-terminal force (PTF), left axis deviation, left ventricular strain, T-wave inversion, and prolonged QRS duration are associated with incremental risk (Hsieh et al. 2005, Morin et al. 2009). On average, electrocardiographic LVH is associated with a hazard ratio (HR) of 1.6 for subsequent cardiovascular events (Chou et al. 2011). Ventricular premature beats Ventricular premature beats (VPBs) and nonsustained VTs have proved to be risk factors for sudden death after myocardial infarction (MI), especially in patients with left ventricular dysfunction (10 or more VPBs per hour in most studies). Patients with nonischemic cardiomyopathy also frequently present with ventricular ectopy, but the relationship between VPBs and SCD is less clear in this population (Goldberger et al. 2008). The suppression of ventricular arrhythmias in post-MI patients with anti-arrhythmic drugs has been associated with increased mortality (Echt et al. 1991). In the absence of structural heart disease, frequent VPBs have reportedly caused tachycardia-related cardiomyopathy (TCM), but fortunately TCM develops only in a minority of subjects (Prystowsky et al. 2012). In the general 40 population, VPBs are associated with traditional risk factors for coronary heart disease (CHD), as well as with greater incidence of CHD events (Massing et al. 2006) and heart failure (Agarwal et al. 2012, Niwano et al. 2009). According to some studies, VPBs also predict increased risk of mortality and SCD in the population (Abdalla et al. 1987, Bikkina et al. 1992, Engel et al. 2007) independently of baseline CHD risk factors and history or symptoms of underlying CHD (Massing et al. 2006). In other studies, however, subjects with no heart disease have shown no mortality risk implications associated with VPBs (Engstrom et al. 1999, Kennedy et al. 1985). Heart rate Elevated resting heart rate has reportedly been a predictor of cardiovascular and non-cardiovascular mortality and SCD in the general population (Benetos et al. 1999, Cooney et al. 2010b, Engel et al. 2007, Gillum et al. 1991, Jouven et al. 2005). Elevated heart rate may only be a marker of an underlying pathophysiological abnormality, but accumulating evidence suggests that heart rate may be an independent risk factor and not just an indicator of risk (Hall & Palmer 2008). This is supported by a study in which reducing the heart rate in the heart failure population with elevated heart rate led to improvement in clinical outcomes (Swedberg et al. 2010). 41 42 3 Purpose of the study This study aimed to elucidate the prevalence and prognostic significance of various electrocardiographic parameters available from the standard 12-lead electrocardiogram in the general population. We were especially interested in the risk of sudden cardiac death associated with these changes. The specific aims of the study were: 1. 2. 3. 4. To assess the significance of prolonged QRS duration and nonspecific intraventricular conduction delay (IVCD) on all-cause mortality, cardiac mortality, and sudden cardiac death (SCD). To study the prognostic significance of wide QRS-T angle, and to evaluate separately the contribution of the QRS-axis and the T-wave axis to the risk of SCD. To evaluate the prevalence and characteristics of T-wave inversions especially on the right precordial leads V1–V3, and to assess the clinical outcome associated with these changes. To assess the clinical significance of prolonged PR interval, or first-degree AV block, in apparently healthy middle-aged subjects. 43 44 4 Participants and methods 4.1 Study population The original study population consisted of 10 899 men and women between 30 and 59 years of age (52% male, mean age 44.0 ± 8.5 years) who had an electrocardiogram recorded as part of the Finnish Social Insurance Institution’s Coronary Heart Disease Study (CHD Study), which was part of the larger prospective Mobile Clinic Health Survey carried out in 35 population groups in Finland. Baseline examinations of the CHD Study were carried out by the Mobile Clinic between 1966 and 1968, and in 1972 (Reunanen et al. 1983). Of the invited subjects, 89.6% participated in the study. The study population comprised 12 population groups from four different geographical areas in Finland with variable mortality and morbidity rates representative of the middle-aged Finnish population. During the initial visit, besides recording a standard 12-lead ECG, the participants were measured and weighed, and had their blood pressure and serum cholesterol measured. Prior to the examination, subjects filled a questionnaire about their previous illnesses, smoking habits, and medication during the past three months. A specially trained nurse then checked the questionnaire to ensure that all the questions were properly answered. 4% of the subjects used AV-nodal blocking medication, mainly digitalis, since betablockers were rare at the time. Any cardiovascular symptoms were documented during the examination using the Rose angina questionnaire (Rose 1962). Between 1973 and 1976, a median of six years after the initial examination, the majority of the participants underwent a repeated evaluation which required another 12-lead ECG. 4.2 Electrocardiographic measurements Thirty minutes after arrival at the Mobile Clinic, a standard 12-lead ECG was recorded with the subject at rest in the supine position. A four-channel recorder (Elema-Schönander Mingograf type 34) was used with a paper speed of 50 mm per second and a calibration of 1 mV per 10 mm, with each ECG sheet containing on average seven to eight QRS complexes. Eight trained technicians supervised by a doctor carried out initial measurements. 45 PR interval, QRS complex, and QT interval were measured for the nearest 10 ms from the bipolar limb lead in which the interval was longest, and the presence or abscence of electrocardiographic LVH according to the Sokolow-Lyon criteria was assessed. Afterwards, the QT interval was corrected for heart rate according to the Bazett’s formula (the QT interval divided by the square root of the R-R interval). The frontal QRS axis and the T axis were determined manually from the six limb leads at 10° intervals using a hexaxial reference system. The QRS-T angle was then calculated as the absolute value of the difference between the QRS axis and the T axis, yielding values between 0° and 180°. Figure 3 demonstrates the measurement of ECG intervals and the QRS-T angle. A B QRS axis 0° I QRS-T angle 90° PR interval QRS interval QT interval T-wave axis aVF Fig. 3. Illustration of the measurement of electrocardiographic intervals (A) and the QRS-T angle (B). To minimize errors in the process, all readers were continuously evaluated using separate ECG material. When compared with the reference values, the intraclass correlation coefficient of r = 0.90 was demonstrated between all readers and the test material for measuring the PR interval, r = 0.97 for measuring the QRS-axis, and r = 0.98 for measuring the T-axis. Later, five physicians independently reevaluated all baseline ECGs for the presence of IVCD, bundle branch blocks, and T-wave inversions, and the duration of the QRS complex was measured were the widest complex was observed. We 46 used the standard criteria for defining LBBB and RBBB, and IVCD was defined as QRS duration ≥ 110 ms not fulfilling the criteria for a partial or complete bundle branch block. We evaluated the presence of inverted T waves of 0.1 mV or more in leads other than aVR, aVL, III, and V1, with more precise characterization of T-wave inversions in the right precordial leads V1 to V3. All ECGs with IVCD, bundle branch block or T wave inversions were later doublechecked, and to further ensure the reliability of the electrocardiographic measurements, 270 ECGs were reassessed for interobserver and intraobserver variation (the kappa-values for QRS duration were 0.66 and 0.68, respectively). 4.3 Follow-up Subjects were followed from the baseline visit that took place between 1966 and 1972 through the end of 2007, with a mean follow-up of 30 ± 11 years. Most of the subjects underwent a reexamination between 1973 and 1976. Fewer than 2% of the subjects were lost as a result of moving abroad, but the survival status of most of these subjects could still be determined. All episodes of hospitalization due to atrial fibrillation, transient ischemic attack (TIA) or stroke, heart failure, coronary heart disease, and ventricular arrhythmias were obtained from the Finnish Hospital Discharge Register, which includes nationwide data on all inpatient episodes in Finland at an individual level. Mortality data were obtained from the Causes of Death Register maintained by Statistics Finland, which records every death in the country. The quality and reliability of these extensive registers has previously been well validated (Pajunen et al. 2005, Rapola et al. 1997). Death certificates were obtained for each deceased individual. Death from cardiac causes was determined according to the appropriate International Classification of Diseases codes (ICD codes). To identify cases of definite or probable arrhythmic deaths, all deaths from cardiac causes were reviewed using death certificates, hospital records, and autopsy reports (if available) according to the definitions presented in the Cardiac Arrhythmia Pilot Study (Greene et al. 1989). According to these criteria, arrhythmia was judged to be the cause of death, defined as spontaneous cessation of respiration and circulation with loss of consciousness, in one of the following situations: (1) witnessed and instantaneous with no new or accelerating symptoms, (2) witnessed and preceded or accompanied by symptoms attributable to myocardial ischemia in the absence of shock or congestive heart failure, (3) witnessed and preceded by symptoms attributable to cardiac arrhythmia (e.g. 47 syncope or near-syncope), or (4) unwitnessed but without evidence of another cause. In the presence of severe congestive heart failure, arrhythmia was judged not to be the cause of death if death from heart failure appeared probable within four months of the fatal episode. During the follow-up, 56.5% of the 10 899 subjects died (n = 6155). Death from cardiovascular causes accounted for 32.2% of all deaths (n = 1980), and 40.5% of this cardiovascular mortality was due to SCD (n = 801). Overall, SCD accounted for 13.0% of all-cause mortality. 4.4 Statistical analysis Continuous data in each study are presented as means ± SD, and categorical variables as a percentage (%) of the participants. The general linear model served to compare the age- and sex-adjusted mean values for continuous variables and the prevalence of categorical variables between the different groups. Hazard ratios and 95% confidence intervals (CI) were calculated with the Cox proportionalhazards model for the primary and secondary endpoints used in each study. The primary adjustments in these models were for age and sex, with further adjustment for covariates that differed between the groups in each study. Age, heart rate, serum cholesterol, systolic blood pressure, QRS duration, JTc duration (QTc - QRS duration) and body mass index (BMI) were added as continuous variables; and sex, smoking, chronotropic medication, cardiovascular disease, ECG signs of coronary artery disease, history of angina pectoris or myocardial infarction, or the presence or absence of electrocardiographic LVH were added as categorical variables in the multivariate models. To further assess differences in mortality between groups, we used the log-rank test to plot Kaplan-Meier survival curves and evaluate their statistical significance. We also generated the receiver operating characteristic (ROC) curves (Study I) and calculated the area under the curve with 95% CI to assess the performance of QRS duration as a risk predictor. P values of less than 0.05 were considered statistically significant. 48 5 Results 5.1 Long-term outcome associated with prolonged QRS duration and nonspecific intraventricular conduction delay (IVCD) Prolonged QRS duration (QRSd) ≥ 110 ms was present in 1.3% (n = 147) of the study participants. The prevalence of partial or complete LBBB was 0.3% (n = 33), and partial or complete RBBB, 0.4% (n = 44). IVCD, defined as QRSd ≥ 110 ms with no bundle branch block or preexcitation, was present in 0.6% (n = 67) of the population. Subjects with IVCD were older and predominantly men, and had lower cholesterol levels than did the rest of the subjects, but we found no differences in the history of prior myocardial infarction or angina pectoris (Table 2). Table 2. Characteristics of the subjects with IVCD at baseline. Characteristics Males (%) No IVCD IVCD (n = 10 862) (n = 67) P value 52.0 93.0 <0.001 44.0 ± 8.5 46.2 ± 9.9 0.03 Current smoker (%) 34.0 26.1 0.14 Total cholesterol (mmol/l) 6.51 6.11 ± 1.34 0.01 25.9 ± 3.9 26.2 ± 3.6 0.59 76 ± 15 77 ± 17 0.49 Age (y) BMI Heart rate (bpm) Systolic blood pressure 138 ± 21 143 ± 28 0.10 QTc duration (ms) 408 ± 28 419 ± 30 0.002 QRS duration (ms) 87 ± 8 112 ± 6 <0.001 LVH (%) 31.4 15.6 0.004 Prior MI (%) 1.1 0.0 0.19 History of angina pectoris (%) 2.3 0.2 0.26 Plus-minus values are means ± SD During the mean follow-up of 30 ± 11 years, subjects with QRSd ≥ 110 ms had higher all-cause mortality (multivariate-adjusted hazard ratio [HR] 1.48, 95% CI 1.22–1.81, P < 0.001), higher cardiovascular mortality (HR 1.94, 95% CI 1.44– 2.63, P < 0.001), and elevated risk of sudden arrhythmic death (HR 2.14, 95% CI 1.38–3.33, P = 0.002). Partial or complete LBBB was associated with increased risk of sudden arrhythmic death (HR 2.71, 95% CI 1.20–6.11, P = 0.04), but RBBB did not predict increased mortality. Subjects with IVCD were at even 49 greater risk of adverse outcomes. IVCD was associated with increased all-cause mortality (HR 2.01, 95% CI 1.52–2.66, P < 0.001), increased cardiovascular mortality (HR 2.53, 95% CI 1.64–3.90, P < 0.001), and even higher risk of sudden arrhythmic death (HR 3.11, 95% CI 1.74–5.54, P = 0.001) (Figure 4). The results remained similar when subjects with no evidence of heart disease were analyzed separately. Also in this group, subjects with QRSd ≥ 110 ms had higher overall mortality (HR 1.34, 95% CI 1.07–1.68, P = 0.02), higher cardiovascular mortality (HR 1.72, 95% CI 1.20–2.46, P = 0.007), and increased risk of sudden arrhythmic death (HR 2.03, 95% CI 1.21–3.41, P = 0.02). In these subjects with no suspected cardiac disease, IVCD was associated with increased all-cause mortality (HR 1.75, 95% CI 1.27–2.40, P = 0.002), cardiovascular mortality (HR 1.87, 95% CI 1.08–3.25, P = 0.04), and the risk of sudden arrhythmic death (HR 2.90, 95% CI 1.49–5.63, P = 0.007). Fig. 4. Kaplan-Meier survival plots for sudden arrhythmic death in subjects with IVCD. 50 5.2 QRS-T angle as a predictor of sudden cardiac death In the middle-aged subjects studied, median QRS axis was 40°, median T-wave axis 30°, and median QRS-T angle 20°. In the study, QRS-T angle 0° to 90°, QRS axis -30° to 90°, and T axis 0° to 90° were considered normal. Of the subjects, 2.1% had a left-axis deviation with QRS-axis ≤ -40°, and 1.3% a right-axis deviation with QRS-axis ≥ 100°. Negative T axis ≤ -10° was present in 4.4% of the subjects, and 0.7% had T axis ≥ 100°. A wide QRS-T angle ≥ 100° occurred in 2.0% (n = 212) of the population. Subjects with QRS-T angle ≥ 100° were older and more often men, were more likely to smoke, and had a higher heart rate and systolic blood pressure. They also had a slightly longer QRS duration, and were more likely to present with inverted T waves, but no difference was noted in the history of prior myocardial infarction or electrocardiographic LVH (Table 3). Table 3. Characteristics of the subjects with wide QRS-T angle at baseline. Characteristics Males (%) Age (y) Current smoker (%) QRS-T angle 0-90° QRS-T angle ≥100° (n = 10 501) (n = 212) P value 52.0 59.6 0.03 43.9 ± 8.4 48.7 ± 8.7 <0.001 33.8 40.0 0.04 Total cholesterol (mmol/l) 6.50 ± 1.31 6.60 ± 1.53 0.26 BMI 25.9 ± 3.8 25.5 ± 4.7 0.09 75 ± 15 81 ± 16 <0.001 <0.001 Heart rate (bpm) Systolic blood pressure 138 ± 21 144 ± 28 QTc duration (ms) 408 ± 27 411 ± 34 0.18 QRS duration (ms) 87 ± 8 90 ± 10 <0.001 Electrocardiographic LVH (%) 31.6 31.7 0.97 Prior MI (%) 1.1 0.1 0.14 History of angina pectoris (%) 2.3 0.0 0.004 Plus-minus values are means ± SD During the long follow-up, subjects with QRS-T angle ≥ 100° had higher allcause mortality (multivariate-adjusted HR 1.57, 95% CI 1.34–1.84, P < 0.001) and a higher risk of SCD (HR 2.26, 95% CI 1.59–3.21, P < 0.001) (Figure 5), but no significant difference was noted in non-arrhythmic cardiac mortality (HR 1.34, 95% CI 0.93–1.92, P = 0.13). The results remained similar when subjects with suspected cardiovascular disease were excluded from the analysis. When the QRS axis and the T axis were analyzed separately, no additional mortality was associated with an abnormal QRS-axis (≤ -40° or ≥ 100°). In contrast, abnormal T 51 axis (≤ -10° or ≥ 100°) was associated with increased risk of all-cause mortality (HR 1.39, 95% CI 1.24–1.55, P < 0.001), non-arrhythmic cardiac mortality (HR 1.87, 95% CI 1.50–2.34, P < 0.001), and SCD (HR 2.13, 95% CI 1.63–2.79, P < 0.001). Fig. 5. Kaplan-Meier survival plots for sudden arrhythmic death in subjects with QRST angle ≥ 100°. 5.3 Prevalence and prognostic significance of T wave inversions Among the study participants, the overall prevalence of inverted T waves in frontal or precordial leads was 1.2% (n = 130). Right precordial T-wave inversions in V1–V3 were present in 0.5% (n = 54) of the entire population. Most of these subjects were women, and their mean heart rate was slower than among the rest of the population. The baseline characteristics of the subjects appear in Table 4. 52 Table 4. Characteristics of the subjects with right precordial T-wave inversions at baseline. Characteristics Males (%) No TWI TWI V1-V3 (n = 10 734) (n = 54) P value 52.4 12.9 <0.001 44.0 ± 8.5 43.6 ± 8.4 0.77 34.0 43.1 0.98 Total cholesterol (mmol/l) 6.50 ± 1.31 6.56 ± 1.78 0.72 BMI Age (y) Current smoker (%) 25.9 ± 3.8 26.0 ± 4.4 0.94 Heart rate (bpm) 76 ± 15 69 ± 13 <0.001 Systolic blood pressure 138 ± 21 135 ± 22 0.18 4.2 2.3 0.48 Chronotropic medication (%) Cardiovascular disease (%) 7.9 14.1 0.08 QTc duration (ms) 408 ± 27 408 ± 31 0.93 QRS duration (ms) 87 ± 8 87 ± 6 0.86 Electrocardiographic LVH (%) 31.4 23.7 0.22 Prior MI (%) 1.1 0.4 0.63 History of angina pectoris (%) 2.3 0.0 0.17 TWI indicates T-wave inversion, plus-minus values are means ± SD For most of these individuals, the T-wave amplitude fell between -0.1mV and 0.4mV, and only four subjects presented with negative T waves of -0.5mV or deeper. In over half of the subjects with right precordial T-wave inversion, right precordial T-wave inversions continued to V4 or beyond. Only one subject with inverted T-waves in V1–V3 also had an epsilon wave, thus fulfilling the diagnostic criteria of ARVC. Most subjects with right precordial T-wave inversion on initial examination also had a control ECG available a median of six years later. In most cases, similar T-wave inversions were still present, and in only 20% of these individuals did T-wave inversions disappear during the follow-up. An example of isolated right precordial T-wave inversion appears in Figure 6. Inverted T-waves confined only to leads other than V1–V3 were present in 0.7% (n = 76) of the population. These subjects were older, had higher blood pressure, and were more likely to have chronotropic medication and cardiovascular disease. They also had a slightly longer QRS duration, but the groups showed no significant differences in sex distribution, LVH, or history of angina pectoris or prior myocardial infarction. 53 I II III V1 aVR V3 aVL V4 aVF V2 V5 V6 Fig. 6. ECG presenting typical mildly inverted right precordial T waves in leads V1–V3. The clinical outcomes associated with right precordial T-wave inversions and Twave inversions in other leads appear in Table 5. No excess in mortality or increase in hospitalizations due to congestive heart failure, ventricular arrhythmia, or coronary artery disease occurred in subjects with right precordial T-wave inversions at V1–V3 or beyond. In contrast, inverted T waves with leads other than V1–V3 were associated with a significant increase in all-cause and cardiovascular mortality, as well as SCD. Moreover, these T-wave inversions predicted heart failure and coronary heart disease hospitalizations. 54 Table 5. Clinical outcomes associated with T-wave inversions in right precordial leads (TWI V1-3) and in other than right precordial leads (TWI other). Outcome No TWI TWI V1-3 P value TWI other P value (n = 10 734) (n = 54) (TWI V1-3) (n = 76) (TWI other) 0.81 1.65 All-cause mortality No. of deaths Age- and sex-adjusted HR 6049 25 1 0.95 (95% CI) Multivariate adjusted HR 59 (0.64–1.41) 1 (95% CI)* 0.93 <0.001 (1.28–2.14) 0.71 (0.61–1.40) 1.49 0.004 (1.15–1.93) Cardiac mortality No. of deaths Age- and sex-adjusted HR 1929 9 1 1.18 (95% CI) Multivariate adjusted HR 31 0.63 (0.61–2.27) 1 (95% CI)* 1.06 2.65 <0.001 (1.86–3.78) 0.88 (0.53–2.12) 2.10 <0.001 (1.46–3.00) Sudden arrhythmic death No. of deaths Age- and sex-adjusted HR 779 2 1 0.76 (95% CI) Multivariate adjusted HR (95% CI)* 14 0.69 (0.19–3.06) 1 0.74 (0.18–2.97) 3.16 <0.001 (1.86–5.36) 0.66 2.38 0.005 (1.39–4.08) * Adjusted for age, sex, heart rate, systolic blood pressure, chronotropic medication, and history of cardiovascular disease 5.4 Prognostic significance of prolonged PR interval in middleaged subjects Altogether 2.1% (n = 222) of the study cohort had a prolonged PR interval > 200 ms, and the prevalence of a more pronounced PR prolongation ≥ 220 ms was 1.1% (n = 123). Only 20 individuals (0.2% of the population) had PR intervals > 240 ms. 70% of the subjects with a prolonged PR interval on initial examination also had a prolonged PR interval at the control ECG a median of six years later. Subjects with a prolonged PR interval were older, more likely to be male, had a higher BMI and lower heart rate, and more often had chronotropic medication and suspected cardiovascular disease than did those with a normal PR interval (Table 6). 55 Table 6. Characteristics of the subjects with prolonged PR interval at baseline. Characteristics Males (%) PR ≤ 200ms PR > 200ms (n = 10 563) (n = 222) P value 51.8 71.9 43.9 ± 8.4 45.4 ± 8.7 0.01 34.1 29.5 0.12 Total cholesterol (mmol/l) 6.51 ± 1.32 6.36 ± 1.34 0.09 BMI 25.9 ± 3.8 26.6 ± 4.0 0.01 Heart rate (bpm) 76 ± 15 71 ± 14 <0.001 Systolic blood pressure 138 ± 21 139 ± 22 0.53 4.2 8.8 <0.001 Age (y) Current smoker (%) Chronotropic medication (%) Cardiovascular disease (%) <0.001 7.9 12.9 0.005 PR interval (ms) 155 ± 20 222 ± 17 <0.001 QTc duration (ms) 408 ± 27 406 ± 28 0.20 QRS duration (ms) 87 ± 8 87 ± 8 0.18 Electrocardiographic LVH (%) 31.3 36.2 0.11 Prior MI (%) 1.1 1.5 0.57 History of angina pectoris (%) 2.3 2.3 0.97 Plus-minus values are means ± SD After adjusting for age and sex, a prolonged PR interval > 200 ms showed no association with increased mortality or hospitalizations due to coronary heart disease, heart failure, atrial fibrillation, TIA or stroke during the follow-up period (Table 7). When subjects with suspected cardiovascular disease, nodal blocking medication, and a prolonged QRS duration ≥ 120 ms were excluded, the results remained unchanged. Using the cutoff PR ≥ 220 ms to define a prolonged PR interval did not affect the results, either (P = non-significant for all endpoints). 56 Table 7. Mortality and risk of hospitalizations associated with a prolonged PR interval. Outcome PR ≤ 200ms PR > 200ms (n = 10 563) (n = 222) P value All-cause mortality No. of deaths 5933 140 Age- and sex-adjusted HR (95% CI) 1 1.04 (0.88–1.23) 0.67 Multivariate-adjusted HR (95% CI) * 1 1.05 (0.89–1.24) 0.56 Cardiac mortality No. of deaths 1904 44 Age- and sex-adjusted HR (95% CI) 1 0.95 (0.71–1.29) 0.75 Multivariate-adjusted HR (95% CI) * 1 0.94 (0.70–1.27) 0.68 Sudden arrhythmic death No. of deaths 766 23 Age- and sex-adjusted HR (95% CI) 1 1.18 (0.78–1.78) 0.46 Multivariate-adjusted HR (95% CI) * 1 1.16 (0.76–1.75) 0.51 Hospitalization for AF No. of deaths 1591 35 Age- and sex-adjusted HR (95% CI) 1 1.10 (0.79–1.55) 0.57 Multivariate-adjusted HR (95% CI) * 1 1.03 (0.74–1.45) 0.85 Hospitalization for TIA or stroke No. of deaths 1877 50 Age- and sex-adjusted HR (95% CI) 1 1.30 (0.98–1.72) 0.08 Multivariate-adjusted HR (95% CI) * 1 1.23 (0.92–1.62) 0.17 Hospitalization for HF No. of cases 1673 43 Age- and sex-adjusted HR (95% CI) 1 1.27 (0.94–1.72) 0.14 Multivariate-adjusted HR (95% CI) * 1 1.22 (0.90–1.65) 0.22 Hospitalization for CHD No. of cases 3465 74 Age- and sex-adjusted HR (95% CI) 1 1.02 (0.81–1.29) 0.84 Multivariate-adjusted HR (95% CI) * 1 0.97 (0.77–1.22) 0.80 * Adjusted for age, sex, BMI, heart rate, chronotropic medication, and cardiovascular disease 57 58 6 Discussion 6.1 QRS duration and IVCD as predictors of sudden cardiac death Prolonged duration of the QRS complex and IVCD in the electrocardiogram have been associated with increased mortality and risk of SCD in the presence of coronary artery disease and heart failure (Teodorescu et al. 2011, Wang et al. 2008, Zimetbaum et al. 2004). In the LIFE study of hypertensive patients with electrocardiographic LVH, prolonged QRS duration was independently predictive of increased risk of SCD (Morin et al. 2009). Previously, however, little has been known of the prognostic significance of prolonged QRS duration and IVCD in individuals with no overt cardiac disease. In Study I, we examined the prognostic impact of prolonged QRS duration and IVCD in the middle-aged general population. Because the optimal cutoff to define prolonged QRS duration in the study population seemed to be 110 ms, we included partial and complete bundle branch blocks and IVCD in the analysis. In the study, we defined IVCD as QRS duration ≥ 110 ms with no bundle branch block-like morphology or ventricular preexcitation. Traditionally, prolonged QRS duration has been defined as QRS ≥ 120 ms, which has also served as the historical cutoff for IVCD (Blackburn et al. 1960). However, recent recommendations for standardizing and interpreting the electrocardiogram defined IVCD similarly to the definition used in our study (Surawicz et al. 2009). The prevalence of prolonged QRS duration and IVCD vary widely depending on the population studied. Among patients with reduced left ventricular ejection fraction hospitalized for heart failure, markedly prolonged QRS duration ≥ 120 ms occurs in up to 40% (Wang et al. 2008). In the general population, however, prolonged QRS duration is rare. Overall prevalence of prolonged QRS duration ≥ 110 ms was 1.3% in our study population. Partial or complete RBBB was present in 0.4% and LBBB in 0.3% of the subjects. IVCD was present in 0.6% of the subjects. During the mean 30-year follow-up, IVCD was associated with a markedly increased risk of SCD in the middle-aged general population studied, together with an increase in all-cause mortality and cardiovascular mortality. The risk of SCD associated with IVCD remained three-fold, however, even after adjusting for other traditional risk factors. Prolonged QRS duration ≥ 110 ms itself (including bundle branch blocks) also proved to be an independent predictor of SCD. The 59 results presented in Study I were recently confirmed in another general population-based sample from Eastern Finland (Kurl et al. 2012). In their population-based sample of over 2000 men with a 19-year follow-up, the risk of SCD increased by 27% for each 10-ms increase in QRS duration with a 2.5-fold higher risk of SCD associated with QRS duration of > 110ms. Several mechanisms could contribute to the adverse prognostic impact of prolonged QRS duration. QRS complex prolongation may be merely a marker of underlying cardiac disease and left ventricular dysfunction, which have a wellestablished relationship with mortality and SCD. The development of a bundle branch block correlates strongly with age, suggesting that it may be a marker of progressive cardiac degeneration associated with aging (Eriksson et al. 1998). Future high-degree atrioventricular block is also strongly associated with the presence of bundle branch block, especially LBBB (Eriksson et al. 2005). Another potential explanation for the adverse prognostic impact of increased QRS duration may be related to markedly abnormal electrical and mechanical activation of the left ventricle. Changes in the ventricular activation sequence in LBBB can induse functional abnormalities manifested by abnormal septal motion as well as a reduction in the global ejection fraction and left ventricular diastolic filling times (Grines et al. 1989, Lee et al. 2003), thereby producing functional mitral regurgitation (Erlebacher & Barbarash 2001). In addition, research has shown left ventricular asynchrony itself to be an independent predictor of severe cardiac events (Bader et al. 2004, Fauchier et al. 2002). Ventricular pacing can also cause abnormalities in ventricular function, and recent research has demonstrated that prolonged paced QRS duration is a major risk factor for future heart failure in patients with continuous right ventricular apical pacing (Chen et al. 2013). On the other hand, the resynchronization of systole with biventricular pacing in patients with wide QRS complex and reduced left ventricular ejection fraction has improved outcomes (Moss et al. 2009). While bundle branch blocks are primarily defects of the conduction system, IVCD is suggestive of a conduction delay in the myocardium outside the HisPurkinje system. This delayed and heterogeneous depolarization can play a direct role in facilitating arrhythmias by acting as a substrate for reentrant ventricular tachycardia (de Bakker et al. 1988, Horwich et al. 2003) and can thus increase one’s risk of SCD. Prolonged QRS duration and IVCD may also indicate the presence of increased myocardial fibrosis and interstitial remodeling. In the Framingham Heart Study population with no cardiac disease, increasing QRS duration was associated with left ventricular mass and dimensions (Dhingra et al. 60 2005). This is supported by an early study that found a positive correlation between the presence or absence of myocardial fibrosis and QRS duration (Mazzoleni et al. 1975). Finally, genetic factors influencing QRS duration are beginning to emerge (Sotoodehnia et al. 2010), so genetic predisposition may also play a role in one’s vulnerability to ventricular arrhythmias in subjects with QRS prolongation. 6.2 QRS-T angle as a risk marker of sudden cardiac death In Study II, we assessed for the first time the mortality risk associated with a wide frontal QRS-T angle in the middle-aged general population. A QRS-T angle ≥ 100° was associated with a more than two-fold risk of SCD, but the risk of nonarrhythmic cardiac death showed no significant increase. This implies that a wide QRS-T angle may be more than a marker of underlying cardiovascular disease, but could also specifically predict one’s vulnerability to lethal arrhythmias. This is supported by a study of subjects with ischemic cardiomyopathy and ICD that demonstrated a markedly higher risk of ventricular arrhythmias associated with a wide QRS-T angle (Borleffs et al. 2009). Another ICD trial with nonischemic cardiomyopathy patients also demonstrated that a wide QRS-T angle predicted increase in mortality and ICD shocks even after adjusting for different clinical variables such as left ventricular ejection fraction and NYHA class (Pavri et al. 2008). In our study, an abnormal QRS axis alone showed no association with adverse outcome. A negative QRS axis is often the result of a left anterior hemiblock (LAHB), which is generally considered a benign finding (Elizari et al. 2007). In contrast, an abnormal T-wave axis seemed to confer most of the increased risk concomitant with a wide QRS-T angle, since it was associated with a similar two-fold risk of SCD. This is in accordance with the results of some previous studies on elderly populations, which have demonstrated that an abnormal T-wave axis is an independent risk factor for cardiac death and nonfatal cardiac events (Kors et al. 1998, Rautaharju et al. 2001). However, an abnormal T-wave axis does not automatically mean abnormal QRS-T angle. In our study population, the prevalence of a wide QRS-T angle ≥ 100° was 2.0%, while that of a negative T-axis ≤ -10°, implying a T-wave inversion in lead aVF, was 4.4%, and that of a positive T-axis ≥ 100°, suggesting a T-wave inversion in lead I, was 0.7%. Thus, many more individuals had an abnormal T-wave axis than an abnormal QRS-T angle. Some of the negative T 61 axes, however, can be regarded as nonspecific changes due to a normal QRS-T angle, which the Minnesota Code already recognized half a century ago. In this electrocardiographic coding system, T-wave inversion in the inferior lead aVF was considered a non-codeable finding, if the QRS complex was not mainly upright in this lead (Macfarlane 2012). 6.3 Prognostic implications of right precordial T-wave inversions Inverted T waves in the right precordial leads beyond V1 are a common finding in children, but these inverted T-waves usually become upright after puberty (Suarez & Suarez 1946). In some individuals, however, this electrocardiographic pattern persists into adulthood, which can pose a clinical problem since right precordial T-wave inversions are also present in various forms of structural cardiac disease potentially capable of causing SCD (Nava et al. 1988, Okada et al. 1994, Thiene et al. 1988). In Study III, we assessed the prevalence and prognostic value of Twave inversions in our study cohort of 30 to 59-year-old subjects from the general population. T-wave inversions in the right precordial leads V1–V3 occurred in 0.5% of the subjects, which is in the range of 0.1% to 3% previously reported by other investigators (Hiss et al. 1960, Marcus 2005, Papadakis et al. 2009, Pelliccia et al. 2007). In most cases, the T-wave inversions were mild to moderate; they were also significantly more common in women than in men. Only 0.03% of the subjects presented with deeply inverted T waves of 5 mm or more. Nearly 80% of the subjects with T-wave inversions in V1–V3 showed inverted T waves in a control ECG taken a median of six years after the initial examination. This suggests that in most cases, T-wave inversion is a constant finding and not due to transient factors capable of causing repolarization abnormalities such as hyperventilation or transient emotional incitement, which can trigger sympathetic activation (Alexopoulos et al. 1996, Atterhog et al. 1981, Blom 1951, Toivonen et al. 1997). An early repolarization pattern in the right precordial leads is especially common in black athletes, and T-wave inversion associated with this electrocardiographic pattern is often considered benign in this setting (Corrado et al. 2009); in the presence of deep inverted T-waves, however, a thorough clinical check-up is recommended, even for athletes (Wilson et al. 2012). In our study population, fewer than 20% of the individuals with inverted T-waves in V1–V3 62 presented with ST-segment elevation in these leads consistent with early repolarization. The right precordial leads can reveal changes in the ECG due to various cardiac conditions such as myocardial ischemia, myocarditis, and hypertrophic or dilated cardiomyopathy, but the electrocardiographic criteria for depolarization and repolarization abnormalities play an especially important role in the diagnosis of ARVC (Marcus et al. 2009, Marcus & Zareba 2009, McKenna et al. 1994). The prevalence of ARVC in the population has been estimated to range from 1 in 1000 to 1 in 5000 (Basso et al. 2009, Peters et al. 2004, Rampazzo et al. 1994), although mutations predisposing to ARVC seem to be much more common in the populace (Lahtinen et al. 2011). According to the new diagnostic criteria for diagnosing ARVC, T-wave inversions in V1–V3 represent major criteria in the category of repolarization abnormalities and epsilon waves major criteria in the category of depolarization abnormalities (Marcus et al. 2010). In ARVC, right precordial T-wave inversions have been reportedly been present in around 48% to 85% of patients, thus reflecting the level of right ventricular involvement (Marcus et al. 2009, Nasir et al. 2004, Nava et al. 1988), whereas the epsilon wave is considered more specific, if less sensitive, for the disease (Cox et al. 2009, Nasir et al. 2004, Uhm et al. 2011). In our study, only one subject with right precordial T-wave inversions showed epsilon waves, thus fulfilling the diagnostic criteria for ARVC, but due to the rarity of the disease and the low sensitivity of these ECG changes, one can draw no direct estimations of the prevalence of ARVC in the Finnish population based on this study. The prognosis associated with inverted T-waves in the right precordial leads V1–V3 was favorable in our study, and mortality and SCD rates did not differ from that of the rest of the population. No differences in hospitalizations for heart failure or ventricular arrhythmias were observed between the groups, either. In contrast, T-wave inversions restricted to other leads than V1–V3 were associated with higher cardiovascular mortality and SCD. This suggests, that mild T-wave inversions in V1–V3 found incidentally in a subject without symptoms, family history or clinical finding suggestive of underlying structural heart disease such as ARVC are likely to be an innocent finding and not an early sign of underlying cardiomyopathy. T-waves in leads other than the right precordial leads, however, may be a sign of underlying cardiac pathology and warrant closer clinical evaluation of these subjects. 63 6.4 Natural history of prolonged PR interval, or first-degree AV block In Study IV, we sought to clarify the prognostic significance of prolonged PR interval in middle-aged subjects. Prolonged PR interval, or first-degree AV-block, has long been regarded as a benign phenomenon (Bexton & Camm 1984). Recently, however, this perception has been challenged by a few studies indicating that prolonged PR interval may be associated with higher risk of atrial fibrillation in some populations (Cheng et al. 2009, Magnani et al. 2013, Soliman et al. 2009). Moreover, PR prolongation has been suggested to predict heart failure hospitalizations (Magnani et al. 2013), pacemaker implantations and allcause mortality (Cheng et al. 2009). In our study, we observed no increase in allcause mortality, cardiovascular mortality, or SCD in subjects with a PR interval > 200ms during the mean 30-year follow-up. These subjects showed no increase in hospitalizations due to heart failure, atrial fibrillation, transient ischemic attack or stroke, either. The results remained unchanged when PR prolongation was defined as PR ≥ 220 ms. Prolonged PR interval is most often the result of delayed conduction in the AV node, although delayed conduction in the atrium and in the His-Purkinje system can also cause PR prolongation. The AV node is strongly influenced by the autonomic nervous system, and over 10% of conditioned athletes, for example, can present with a prolonged PR interval due to increased vagal tone and decreased sympathetic tone (Corrado et al. 2009, Pelliccia et al. 2000). The PR interval is generally longer in men and prolongs further with age (Magnani et al. 2011). Electrophysiological studies have demonstrated that aging results in increased conduction times and refractoriness in the atria (Kistler et al. 2004), and in decelerated conduction of the AV node and the more distal conduction system (Taneja et al. 2001). Changes in the atrioventricular conduction associated with vagal tone and aging may explain why the results of most previous studies and our present work conducted on young or middle-aged subjects seem to differ from those of some recent publications. In the Framingham population, a PR interval > 200 ms predicted all-cause mortality with an unadjusted hazard ratio of 2.7 and a multivariate-adjusted hazard ratio of 1.4. The adjusted risk of atrial fibrillation was two-fold, and the risk of pacemaker implantation was three-fold higher than that of the rest of the population (Cheng et al. 2009). However, the mean age of the subjects with prolonged PR interval was 55 years –almost 10 years more than the average age 64 of the rest of the cohort, which might explain why the outcomes in this study differed from those of previous reports. Similar results have also been obtained from patients with stable coronary artery disease, in whom a PR interval ≥ 220 ms was associated with heart failure and cardiovascular death (Crisel et al. 2011). A prolonged PR interval can be a marker of more extensive calcification and fibrosis of the conduction system (Lev 1964), and especially in the elderly, this could forecast more severe disturbances of the AV conduction influencing the prognosis. Degenerative changes and fibrosis of the sinus node and the atria associated with aging could also predispose an individual to a higher risk of atrial arrhythmias. Moreover, a prolonged PR interval can disturb left ventricular filling and cause diastolic mitral regurgitation (Appleton et al. 1991, Barold et al. 2006, Schnittger et al. 1988), which may be hemodynamically important, especially in older individuals with systolic or diastolic ventricular dysfunction. Taken together, in the middle-aged population with no cardiovascular disease, a prolonged PR interval seems to be an innocent electrocardiographic finding with no prognostic implications. However, this may not be true in subjects with prolonged QRS duration, which implies that conduction delay does not occur solely in the AV node but is due to disturbed conduction in the His-Purkinje system. In the elderly, on the other hand, PR prolongation is more likely to be associated with structural cardiac disease and a risk of adverse outcome, and thus requires a more meticulous approach. 6.5 Future aspects of electrocardiography in SCD prevention The 12-lead ECG is an invaluable tool in the diagnosis of a variety of cardiac diseases, including arrhythmias, myocardial ischemia, and hereditary ion channel disorders, and can also prove helpful in identifying individuals with underlying cardiomyopathy. The role of ECG as a screening method to prevent SCD in susceptible individuals has recently sparked great interest. A special group that may potentially benefit from the early detection of underlying cardiac abnormalities is athletes engaged in competitive sports. A variety of clinical, experimental and epidemiological evidence suggests that regular physical activity decreases one’s risk of cardiovascular events and SCD, but on the other hand, vigorous exercise transiently increases one’s risk of acute coronary events and potentially lethal ventricular arrhythmias in susceptible individuals (Thompson et al. 2007). Among young athletes, cardiomyopathies, coronary anomalies, and premature coronary artery disease are among the most 65 important cardiac pathologies responsible for SCD. In Italy, preparticipation screening of athletes that includes the 12-lead ECG has already began three decades ago and has led to a marked decline in the incidence of SCD, mainly due to fewer sudden deaths from cardiomyopathies (Corrado et al. 2006). This encouraging experience has led the European Society of Cardiology to recommend electrocardiographic screening for all young athletes involved in competitive sports (Corrado et al. 2005). The preparticipation screening protocol is currently implemented in many countries, although the rate of false positive ECGs in athletes (i.e. electrocardiographic changes leading to further investigations revealing no underlying cardiac pathology) has reportedly risen to as high as 9% (Corrado et al. 2011). However, the efficacy and cost-benefit of the systematic screening of athletes has recently come under discussion (Halkin et al. 2012, Steinvil et al. 2011). Among individuals over 35 years of age, coronary artery disease is by far the most common cause of SCD (Corrado et al. 2011); thus, traditional risk factors such as older age, male sex, high blood pressure, abnormal serum low- and highdensity lipoprotein (LDL and HDL) concentrations, smoking, and diabetes can prove helpful in predicting future cardiovascular risk (Greenland et al. 2003a). However, although these factors may be associated with SCD, their predictive value is low; consequently, interest has grown in additional methods such as resting and exercise ECG in order to assist in risk stratification and to guide therapies in reducing the risk of future cardiovascular events (O'Malley & Redberg 2010). A wide range of electrocardiographic abnormalities such as prolonged QRS duration, LBBB, IVCD, LVH, ER, QTc-interval, wide QRS-T angle and even minor ST-T abnormalities have been associated with increased risk of cardiovascular death (Aro et al. 2011, Aro et al. 2012, Daviglus et al. 1999, Greenland et al. 2003a, Tikkanen et al. 2009). However, effect on clinical outcomes obtained with the screening of asymptomatic individuals with resting ECGs versus no screening remains poorly demonstrated. The problem of screening is highlighted by a recent study that randomized type II diabetics for screening of asymptomatic coronary artery disease. It is well established that diabetic patients are at higher risk of coronary events, but even in this high-risk population, a randomized trial failed to show any benefit from a systematic search for ischemia in asymptomatic individuals (Young et al. 2009). Moreover, evidence on how identifying high-risk persons with ECG affects the use of 66 medical and other interventions in reducing cardiovascular risk is inadequate (Chou et al. 2011). Another problem with systematic electrocardiographic screening of the population is that the majority of coronary events occur in the absence of prior ECG abnormalities, so the sensitivity and specificity of the screening would likely be low (Corrado et al. 2011). In their recent clinical guidelines, the U.S. Preventive Services Task Force (USPSTF) recommended against routine electrocardiographic screening of lowrisk asymptomatic adults for the prediction of cardiac events (Moyer & U.S. Preventive Services Task Force 2012). In clinical practice, however, innumerable ECGs are recorded every day for a wide variety of reasons from individuals with and without overt cardiac disease. When an abnormal electrocardiographic pattern is encountered in a routine ECG, its clinical significance is of great interest to both the physician and the patient. Although the evidence may not be sufficiently robust to recommend systematic ECG screening, it seems reasonable to assume that an individual with an electrocardiographic pattern associated with greater risk will benefit from further clinical evaluation and closer follow-up. The positive predictive accuracy of each of these ECG risk markers remains relatively low, so further efforts are still needed to improve the risk stratification of our patients and to develop strategies for SCD prevention. 6.6 Potential limitations of the studies The number of deaths classified as SCD varies significantly between different studies due to the different definitions they used to define SCD and whether the arrhythmia itself was documented. In our studies, we used a widely accepted criterion to define probable arrhythmic death (Greene et al. 1989). This endpoint, however, is somewhat biased. Even though rapid ventricular arrhythmias are considered the most common mechanism of SCD, many other conditions than arrhythmias can evolve rapidly and lead to sudden death. In fact, studies of patients with ICD have demonstrated that many deaths defined as sudden were not due to arrhythmias, but to other cardiac and noncardiac causes (Grubman et al. 1998, Pratt et al. 1996). Another potential limitation in the present studies relates to the manual measurements of the different electrocardiographic parameters. The use of automated ECG machines could have yielded more precise information on the various intervals in the electrocardiogram even though great care was taken to ensure the accuracy and reproducibility of the data. The PR interval is itself a 67 dynamic interval, and measuring it to the nearest millisecond rather than in 10-ms intervals is unlikely to significantly change the outcomes. The automated measurement of QRS duration, however, may have altered the results. Our method of measuring QRS duration to the nearest 10 ms in Study I would, if anything, result in a conservative bias and does not explain the significant survival difference observed based on QRS duration. The same holds true for the estimation of the frontal QRS-T angle in Study II. The demographics of the Finnish population have changed considerably over the past 40 years, with significant changes in lifestyle and a reduction of the cardiovascular risk factors and mortality (Cooney et al. 2010a, Harald et al. 2008), as well as profound changes in the diagnosis and treatment of various cardiovascular conditions. Consequently, some of the observations presented here may prove inapplicable in more contemporary population. The baseline visit in 1966–1972 entailed a detailed cardiovascular risk assessment that included routine laboratory samples; the participants were carefully interviewed for the presence of chest pain, dyspnea, and peripheral artery disease, but underwent no further diagnostic testing, such as an exercise test or echocardiography. Consequently, some of the subjects may have had an underlying cardiac disease undiscovered during the initial workup. This resembles normal clinical practice, however, and makes the results of the studies generalizable to everyday clinical work, in which electrocardiography still holds an important place 110 years after its inception. 68 7 Conclusions The aim behind this thesis was to identify electrocardiographic patterns that may predict SCD in the general population, as well as to recognize patterns that have a relatively benign prognosis. The results of Study I show that in the middle-aged general population, QRS duration ≥ 110 ms is a relatively rare finding, with a prevalence of 1.3%, but is associated with increased mortality. IVCD, defined as QRS ≥ 110 ms with no partial or complete bundle branch block, was present in 0.6% of the subjects. The risk of cardiac mortality associated with IVCD was more than two-fold, and the risk of SCD, three-fold higher than that of the rest of the population. In Study II, we examined the value of the frontal QRS-T angle, the angle between the QRS-axis, representing the direction of depolarization, and the Taxis, representing the vector of repolarization in the frontal plane, in SCD risk stratification in the same population. We demonstrated that a wide QRS-T angle ≥ 100° was associated with increased mortality and doubled one’s risk of SCD during the follow-up period. This seemed to result mostly from an abnormal Twave axis, since the QRS axis had no effect on prognosis. In Study III, we investigated the prognostic implications of T wave inversions. Inverted T waves in the right precordial leads V1–V3 often manifest in children and adolescents, but when they persist into adulthood, these changes may raise a suspicion of underlying cardiac disease. In the middle-aged population studied, the prevalence of right precordial T-wave inversions was 0.5%. The main finding of this study was that these T-wave inversions showed no association with reduced survival or SCD. In contrast, inverted T waves in leads other than the right precordial leads predicted increased cardiovascular mortality. Study IV aimed to examine the effect of the prolonged PR interval, or firstdegree AV block, on mortality and morbidity, since conflicting results exist on the clinical significance of this phenomenon. In the middle-aged population studied, a PR interval > 200 ms occured in 2.1% of the subjects, but no increase in mortality or hospitalizations due to AF, stroke, or heart failure was associated with PR prolongation. Taken together, IVCD or wide QRS-T can represent early signs of underlying cardiac pathology or vulnerability to ventricular arrhythmias on even an asymptomatic individual, and their presence on an ECG should lead to closer clinical evaluation and follow-up. On the other hand, in the middle-aged subjects with no suspected cardiac pathology, a prolonged PR interval and right precordial 69 T-wave inversions seem to be innocent findings that show no association with adverse outcome. 70 References Abdalla IS, Prineas RJ, Neaton JD, Jacobs DR,Jr & Crow RS. (1987) Relation between ventricular premature complexes and sudden cardiac death in apparently healthy men. Am J Cardiol 60(13): 1036–1042. Agarwal SK, Simpson RJ,Jr, Rautaharju P, Alonso A, Shahar E, Massing M, Saba S & Heiss G. (2012) Relation of ventricular premature complexes to heart failure (from the Atherosclerosis Risk In Communities [ARIC] Study). Am J Cardiol 109(1): 105–109. Alexopoulos D, Christodoulou J, Toulgaridis T, Sitafidis G, Manias O, Hahalis G & Vagenakis AG. (1996) Repolarization abnormalities with prolonged hyperventilation in apparently healthy subjects: incidence, mechanisms and affecting factors. Eur Heart J 17(9): 1432–1437. Algra A, Tijssen JG, Roelandt JR, Pool J & Lubsen J. (1991) QTc prolongation measured by standard 12-lead electrocardiography is an independent risk factor for sudden death due to cardiac arrest. Circulation 83(6): 1888–1894. Anttonen O, Junttila MJ, Rissanen H, Reunanen A, Viitasalo M & Huikuri HV. (2007) Prevalence and prognostic significance of short QT interval in a middle-aged Finnish population. Circulation 116(7): 714–720. Antzelevitch C, Yan GX & Viskin S. (2011) Rationale for the use of the terms J-wave syndromes and early repolarization. J Am Coll Cardiol 57(15): 1587–1590. Appleton CP, Basnight MA & Gonzalez MS. (1991) Diastolic mitral regurgitation with atrioventricular conduction abnormalities: relation of mitral flow velocity to transmitral pressure gradients in conscious dogs. J Am Coll Cardiol 18(3): 843–849. Aro AL, Anttonen O, Tikkanen JT, Junttila MJ, Kerola T, Rissanen HA, Reunanen A & Huikuri HV. (2011) Intraventricular conduction delay in a standard 12-lead electrocardiogram as a predictor of mortality in the general population. Circ Arrhythm Electrophysiol 4(5): 704–710. Aro AL, Huikuri HV, Tikkanen JT, Junttila MJ, Rissanen HA, Reunanen A & Anttonen O. (2012) QRS-T angle as a predictor of sudden cardiac death in a middle-aged general population. Europace 14(6): 872–876. Atterhog JH, Eliasson K & Hjemdahl P. (1981) Sympathoadrenal and cardiovascular responses to mental stress, isometric handgrip, and cold pressor test in asymptomatic young men with primary T wave abnormalities in the electrocardiogram. Br Heart J 46(3): 311–319. Atterhog JH & Loogna E. (1977) P-R interval in relation to heart rate during exercise and the influence of posture and autonomic tone. J Electrocardiol 10(4): 331–336. Bader H, Garrigue S, Lafitte S, Reuter S, Jais P, Haissaguerre M, Bonnet J, Clementy J & Roudaut R. (2004) Intra-left ventricular electromechanical asynchrony. A new independent predictor of severe cardiac events in heart failure patients. J Am Coll Cardiol 43(2): 248–256. 71 Barold SS, Ilercil A, Leonelli F & Herweg B. (2006) First-degree atrioventricular block. Clinical manifestations, indications for pacing, pacemaker management & consequences during cardiac resynchronization. J Interv Card Electrophysiol 17(2): 139–152. Baruteau AE, Behaghel A, Fouchard S, Mabo P, Schott JJ, Dina C, Chatel S, Villain E, Thambo JB, Marcon F, Gournay V, Rouault F, Chantepie A, Guillaumont S, Godart F, Martins RP, Delasalle B, Bonnet C, Fraisse A, Schleich JM, Lusson JR, Dulac Y, Daubert JC, Le Marec H & Probst V. (2012) Parental electrocardiographic screening identifies a high degree of inheritance for congenital and childhood nonimmune isolated atrioventricular block. Circulation 126(12): 1469–1477. Baslaib F, Alkaabi S, Yan AT, Yan RT, Dorian P, Nanthakumar K, Casanova A, Goodman SG & Canadian Acute Coronary Syndrome Registry Investigators. (2010) QRS prolongation in patients with acute coronary syndromes. Am Heart J 159(4): 593–598. Basso C, Corrado D, Marcus FI, Nava A & Thiene G. (2009) Arrhythmogenic right ventricular cardiomyopathy. Lancet 373(9671): 1289–1300. Bayes de Luna A, Coumel P & Leclercq JF. (1989) Ambulatory sudden cardiac death: mechanisms of production of fatal arrhythmia on the basis of data from 157 cases. Am Heart J 117(1): 151–159. Benetos A, Rudnichi A, Thomas F, Safar M & Guize L. (1999) Influence of heart rate on mortality in a French population: role of age, gender, and blood pressure. Hypertension 33(1): 44–52. Benito B, Guasch E, Rivard L & Nattel S. (2010) Clinical and mechanistic issues in early repolarization of normal variants and lethal arrhythmia syndromes. J Am Coll Cardiol 56(15): 1177–1186. Bexton RS & Camm AJ. (1984) First degree atrioventricular block. Eur Heart J 5 Suppl A: 107–109. Bikkina M, Larson MG & Levy D. (1992) Prognostic implications of asymptomatic ventricular arrhythmias: the Framingham Heart Study. Ann Intern Med 117(12): 990– 996. Blackburn H, Keys A, Simonson E, Rautaharju P & Punsar S. (1960) The electrocardiogram in population studies. A classification system. Circulation 21: 1160–1175. Blackburn H, Taylor HL & Keys A. (1970) Coronary heart disease in seven countries. XVI. The electrocardiogram in prediction of five-year coronary heart disease incidence among men aged forty through fifty-nine. Circulation 41(4 Suppl): I154–61. Blom GE. (1951) A review of electrocardiographic changes in emotional states, with a clinical note on electrocardiograms of 193 psychotic patients. J Nerv Ment Dis 113(4): 283–300. Bongioanni S, Bianchi F, Migliardi A, Gnavi R, Pron PG, Casetta M & Conte MR. (2007) Relation of QRS duration to mortality in a community-based cohort with hypertrophic cardiomyopathy. Am J Cardiol 100(3): 503–506. 72 Borleffs CJ, Scherptong RW, Man SC, van Welsenes GH, Bax JJ, van Erven L, Swenne CA & Schalij MJ. (2009) Predicting ventricular arrhythmias in patients with ischemic heart disease: clinical application of the ECG-derived QRS-T angle. Circ Arrhythm Electrophysiol 2(5): 548–554. Breithardt G & Breithardt OA. (2012) Left bundle branch block, an old-new entity. J Cardiovasc Transl Res 5(2): 107–116. Burgess MJ. (1979) Relation of ventricular repolarization to electrocardiographic T waveform and arrhythmia vulnerability. Am J Physiol 236(3): H391–402. Bussink BE, Holst AG, Jespersen L, Deckers JW, Jensen GB & Prescott E. (2013) Right bundle branch block: prevalence, risk factors, and outcome in the general population: results from the Copenhagen City Heart Study. Eur Heart J 34: 138–146. Chen S, Yin Y, Lan X, Liu Z, Ling Z, Su L, Kiuchi MG, Li X, Zhong B, Krucoff MW & The PREDICT-Heart Failure study international group. (2013) Paced QRS duration as a predictor for clinical heart failure events during right ventricular apical pacing in patients with idiopathic complete atrioventricular block: results from an observational cohort study (PREDICT-HF). Eur J Heart Fail 15(3): 352–359. Cheng S, Keyes MJ, Larson MG, McCabe EL, Newton-Cheh C, Levy D, Benjamin EJ, Vasan RS & Wang TJ. (2009) Long-term outcomes in individuals with prolonged PR interval or first-degree atrioventricular block. JAMA 301(24): 2571–2577. Cheng S, Larson MG, Keyes MJ, McCabe EL, Newton-Cheh C, Levy D, Benjamin EJ, Vasan RS & Wang TJ. (2010) Relation of QRS width in healthy persons to risk of future permanent pacemaker implantation. Am J Cardiol 106(5): 668–672. Chou R, Arora B, Dana T, Fu R, Walker M & Humphrey L. (2011) Screening asymptomatic adults with resting or exercise electrocardiography: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 155(6): 375– 385. Chugh SS, Jui J, Gunson K, Stecker EC, John BT, Thompson B, Ilias N, Vickers C, Dogra V, Daya M, Kron J, Zheng ZJ, Mensah G & McAnulty J. (2004) Current burden of sudden cardiac death: multiple source surveillance versus retrospective death certificate-based review in a large U.S. community. J Am Coll Cardiol 44(6): 1268– 1275. Chugh SS, Reinier K, Singh T, Uy-Evanado A, Socoteanu C, Peters D, Mariani R, Gunson K & Jui J. (2009a) Determinants of prolonged QT interval and their contribution to sudden death risk in coronary artery disease: the Oregon Sudden Unexpected Death Study. Circulation 119(5): 663–670. Chugh SS, Uy-Evanado A, Teodorescu C, Reinier K, Mariani R, Gunson K & Jui J. (2009b) Women have a lower prevalence of structural heart disease as a precursor to sudden cardiac arrest: The Ore-SUDS (Oregon Sudden Unexpected Death Study). J Am Coll Cardiol 54(22): 2006–2011. Cooney MT, Vartiainen E, Laatikainen T, Joulevi A, Dudina A & Graham I. (2010a) Simplifying cardiovascular risk estimation using resting heart rate. Eur Heart J 31(17): 2141–2147. 73 Cooney MT, Vartiainen E, Laatikainen T, Juolevi A, Dudina A & Graham IM. (2010b) Elevated resting heart rate is an independent risk factor for cardiovascular disease in healthy men and women. Am Heart J 159(4): 612–619.e3. Corrado D, Basso C, Pavei A, Michieli P, Schiavon M & Thiene G. (2006) Trends in sudden cardiovascular death in young competitive athletes after implementation of a preparticipation screening program. JAMA 296(13): 1593–1601. Corrado D, Biffi A, Basso C, Pelliccia A & Thiene G. (2009) 12-lead ECG in the athlete: physiological versus pathological abnormalities. Br J Sports Med 43(9): 669–676. Corrado D, Pelliccia A, Bjornstad HH, Vanhees L, Biffi A, Borjesson M, PanhuyzenGoedkoop N, Deligiannis A, Solberg E, Dugmore D, Mellwig KP, Assanelli D, Delise P, van-Buuren F, Anastasakis A, Heidbuchel H, Hoffmann E, Fagard R, Priori SG, Basso C, Arbustini E, Blomstrom-Lundqvist C, McKenna WJ, Thiene G & Study Group of Sport Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. (2005) Cardiovascular pre-participation screening of young competitive athletes for prevention of sudden death: proposal for a common European protocol. Consensus Statement of the Study Group of Sport Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Eur Heart J 26(5): 516–524. Corrado D, Schmied C, Basso C, Borjesson M, Schiavon M, Pelliccia A, Vanhees L & Thiene G. (2011) Risk of sports: do we need a pre-participation screening for competitive and leisure athletes? Eur Heart J 32(8): 934–944. Cox MG, van der Smagt JJ, Noorman M, Wiesfeld AC, Volders PG, van Langen IM, Atsma DE, Dooijes D, Houweling AC, Loh P, Jordaens L, Arens Y, Cramer MJ, Doevendans PA, van Tintelen JP, Wilde AA & Hauer RN. (2010) Arrhythmogenic right ventricular dysplasia/cardiomyopathy diagnostic task force criteria: impact of new task force criteria. Circ Arrhythm Electrophysiol 3(2): 126–133. Cox MG, van der Smagt JJ, Wilde AA, Wiesfeld AC, Atsma DE, Nelen MR, Rodriguez LM, Loh P, Cramer MJ, Doevendans PA, van Tintelen JP, de Bakker JM & Hauer RN. (2009) New ECG criteria in arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circ Arrhythm Electrophysiol 2(5): 524–530. Crisel RK, Farzaneh-Far R, Na B & Whooley MA. (2011) First-degree atrioventricular block is associated with heart failure and death in persons with stable coronary artery disease: data from the Heart and Soul Study. Eur Heart J 32(15): 1875–1880. Cuddy TE & Tate RB. (2006) Sudden unexpected cardiac death as a function of time since the detection of electrocardiographic and clinical risk factors in apparently healthy men: the Manitoba Follow-Up Study, 1948 to 2004. Can J Cardiol 22(3): 205–211. Cupples LA, Gagnon DR & Kannel WB. (1992) Long- and short-term risk of sudden coronary death. Circulation 85(1 Suppl): I11–8. 74 Daviglus ML, Liao Y, Greenland P, Dyer AR, Liu K, Xie X, Huang CF, Prineas RJ & Stamler J. (1999) Association of nonspecific minor ST-T abnormalities with cardiovascular mortality: the Chicago Western Electric Study. JAMA 281(6): 530– 536. de Bakker JM, van Capelle FJ, Janse MJ, Wilde AA, Coronel R, Becker AE, Dingemans KP, van Hemel NM & Hauer RN. (1988) Reentry as a cause of ventricular tachycardia in patients with chronic ischemic heart disease: electrophysiologic and anatomic correlation. Circulation 77(3): 589–606. de Torbal A, Kors JA, van Herpen G, Meij S, Nelwan S, Simoons ML & Boersma E. (2004) The electrical T-axis and the spatial QRS-T angle are independent predictors of long-term mortality in patients admitted with acute ischemic chest pain. Cardiology 101(4): 199–207. Deo R & Albert CM. (2012) Epidemiology and genetics of sudden cardiac death. Circulation 125(4): 620–637. Derval N, Shah A & Jais P. (2011) Definition of early repolarization: a tug of war. Circulation 124(20): 2185–2186. Desai AD, Yaw TS, Yamazaki T, Kaykha A, Chun S & Froelicher VF. (2006) Prognostic Significance of Quantitative QRS Duration. Am J Med 119(7): 600–606. Dhingra R, Ho Nam B, Benjamin EJ, Wang TJ, Larson MG, D'Agostino RB S, Levy D & Vasan RS. (2005) Cross-sectional relations of electrocardiographic QRS duration to left ventricular dimensions: the Framingham Heart Study. J Am Coll Cardiol 45(5): 685–689. Dilaveris PE, Farbom P, Batchvarov V, Ghuran A & Malik M. (2001) Circadian behavior of P-wave duration, P-wave area, and PR interval in healthy subjects. Ann Noninvasive Electrocardiol 6(2): 92–97. Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias-Manno D, Barker AH, Arensberg D, Baker A, Friedman L & Greene HL. (1991) Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med 324(12): 781–788. Elizari MV, Acunzo RS & Ferreiro M. (2007) Hemiblocks revisited. Circulation 115(9): 1154–1163. Engel G, Cho S, Ghayoumi A, Yamazaki T, Chun S, Fearon WF & Froelicher VF. (2007) Prognostic significance of PVCs and resting heart rate. Ann Noninvasive Electrocardiol 12(2): 121–129. Engstrom G, Hedblad B, Janzon L & Juul-Moller S. (1999) Ventricular arrhythmias during 24-h ambulatory ECG recording: incidence, risk factors and prognosis in men with and without a history of cardiovascular disease. J Intern Med 246(4): 363–372. 75 Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA,3rd, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO, Smith SC,Jr, Jacobs AK, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Faxon DP, Halperin JL, Hiratzka LF, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura RA, Ornato JP, Page RL, Riegel B, Tarkington LG, Yancy CW, American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices), American Association for Thoracic Surgery & Society of Thoracic Surgeons. (2008) ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices): developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation 117(21): e350–408. Erikssen J & Otterstad JE. (1984) Natural course of a prolonged PR interval and the relation between PR and incidence of coronary heart disease. A 7-year follow-up study of 1832 apparently healthy men aged 40–59 years. Clin Cardiol 7(1): 6–13. Eriksson P, Hansson PO, Eriksson H & Dellborg M. (1998) Bundle-branch block in a general male population: the study of men born 1913. Circulation 98(22): 2494–2500. Eriksson P, Wilhelmsen L & Rosengren A. (2005) Bundle-branch block in middle-aged men: risk of complications and death over 28 years. The Primary Prevention Study in Goteborg, Sweden. Eur Heart J 26(21): 2300–2306. Erlebacher JA & Barbarash S. (2001) Intraventricular conduction delay and functional mitral regurgitation. Am J Cardiol 88(1): A7, 83–6. Estes NA,3rd. (2011) Predicting and preventing sudden cardiac death. Circulation 124(5): 651–656. Fahy GJ, Pinski SL, Miller DP, McCabe N, Pye C, Walsh MJ & Robinson K. (1996) Natural history of isolated bundle branch block. Am J Cardiol 77(14): 1185–1190. Fauchier L, Marie O, Casset-Senon D, Babuty D, Cosnay P & Fauchier JP. (2002) Interventricular and intraventricular dyssynchrony in idiopathic dilated cardiomyopathy: a prognostic study with fourier phase analysis of radionuclide angioscintigraphy. J Am Coll Cardiol 40(11): 2022–2030. Fernández-Lozano I & Brugada J. (2013) Right bundle branch block: are we looking in the right direction? Eur Heart J 34: 86–88. Fishman GI, Chugh SS, Dimarco JP, Albert CM, Anderson ME, Bonow RO, Buxton AE, Chen PS, Estes M, Jouven X, Kwong R, Lathrop DA, Mascette AM, Nerbonne JM, O'Rourke B, Page RL, Roden DM, Rosenbaum DS, Sotoodehnia N, Trayanova NA & Zheng ZJ. (2010) Sudden cardiac death prediction and prevention: report from a National Heart, Lung, and Blood Institute and Heart Rhythm Society Workshop. Circulation 122(22): 2335–2348. 76 Freedman RA, Alderman EL, Sheffield LT, Saporito M & Fisher LD. (1987) Bundle branch block in patients with chronic coronary artery disease: angiographic correlates and prognostic significance. J Am Coll Cardiol 10(1): 73–80. Gang UJ, Jons C, Jorgensen RM, Abildstrom SZ, Haarbo J, Messier MD, Huikuri HV, Thomsen PE & CARISMA investigators. (2010) Heart rhythm at the time of death documented by an implantable loop recorder. Europace 12(2): 254–260. Gillum RF, Makuc DM & Feldman JJ. (1991) Pulse rate, coronary heart disease, and death: the NHANES I Epidemiologic Follow-up Study. Am Heart J 121(1 Pt 1): 172–177. Giustetto C, Di Monte F, Wolpert C, Borggrefe M, Schimpf R, Sbragia P, Leone G, Maury P, Anttonen O, Haissaguerre M & Gaita F. (2006) Short QT syndrome: clinical findings and diagnostic-therapeutic implications. Eur Heart J 27(20): 2440–2447. Go AS, Barron HV, Rundle AC, Ornato JP & Avins AL. (1998) Bundle-branch block and in-hospital mortality in acute myocardial infarction. National Registry of Myocardial Infarction 2 Investigators. Ann Intern Med 129(9): 690–697. Goldberger JJ, Cain ME, Hohnloser SH, Kadish AH, Knight BP, Lauer MS, Maron BJ, Page RL, Passman RS, Siscovick D, Siscovick D, Stevenson WG, Zipes DP, American Heart Association, American College of Cardiology Foundation & Heart Rhythm Society. (2008) American Heart Association/American College of Cardiology Foundation/Heart Rhythm Society scientific statement on noninvasive risk stratification techniques for identifying patients at risk for sudden cardiac death: a scientific statement from the American Heart Association Council on Clinical Cardiology Committee on Electrocardiography and Arrhythmias and Council on Epidemiology and Prevention. Circulation 118(14): 1497–1518. Goldenberg I & Moss AJ. (2008) Long QT syndrome. J Am Coll Cardiol 51(24): 2291– 2300. Goldenberg I, Vyas AK, Hall WJ, Moss AJ, Wang H, He H, Zareba W, McNitt S, Andrews ML & MADIT-II Investigators. (2008) Risk stratification for primary implantation of a cardioverter-defibrillator in patients with ischemic left ventricular dysfunction. J Am Coll Cardiol 51(3): 288–296. Greene HL, Richardson DW, Barker AH, Roden DM, Capone RJ, Echt DS, Friedman LM, Gillespie MJ, Hallstrom AP & Verter J. (1989) Classification of deaths after myocardial infarction as arrhythmic or nonarrhythmic (the Cardiac Arrhythmia Pilot Study). Am J Cardiol 63(1): 1–6. Greenland P, Knoll MD, Stamler J, Neaton JD, Dyer AR, Garside DB & Wilson PW. (2003a) Major risk factors as antecedents of fatal and nonfatal coronary heart disease events. JAMA 290(7): 891–897. Greenland P, Xie X, Liu K, Colangelo L, Liao Y, Daviglus ML, Agulnek AN & Stamler J. (2003b) Impact of minor electrocardiographic ST-segment and/or T-wave abnormalities on cardiovascular mortality during long-term follow-up. Am J Cardiol 91(9): 1068–1074. 77 Greve AM, Gerdts E, Boman K, Gohlke-Baerwolf C, Rossebo AB, Devereux RB, Kober L, Ray S, Willenheimer R & Wachtell K. (2012) Impact of QRS duration and morphology on the risk of sudden cardiac death in asymptomatic patients with aortic stenosis: the SEAS (Simvastatin and Ezetimibe in Aortic Stenosis) Study. J Am Coll Cardiol 59(13): 1142–1149. Grines CL, Bashore TM, Boudoulas H, Olson S, Shafer P & Wooley CF. (1989) Functional abnormalities in isolated left bundle branch block. The effect of interventricular asynchrony. Circulation 79(4): 845–853. Grubman EM, Pavri BB, Shipman T, Britton N & Kocovic DZ. (1998) Cardiac death and stored electrograms in patients with third-generation implantable cardioverterdefibrillators. J Am Coll Cardiol 32(4): 1056–1062. Haataja P, Nikus K, Kähönen M, Huhtala H, Nieminen T, Jula A, Reunanen A, Salomaa V, Sclarovsky S, Nieminen MS & Eskola M. (2012) Prevalence of ventricular conduction blocks in the resting electrocardiogram in a general population: The Heath 2000 Survey. Int J Cardiol [Epub ahead of print]. Haissaguerre M, Derval N, Sacher F, Jesel L, Deisenhofer I, de Roy L, Pasquie JL, Nogami A, Babuty D, Yli-Mayry S, De Chillou C, Scanu P, Mabo P, Matsuo S, Probst V, Le Scouarnec S, Defaye P, Schlaepfer J, Rostock T, Lacroix D, Lamaison D, Lavergne T, Aizawa Y, Englund A, Anselme F, O'Neill M, Hocini M, Lim KT, Knecht S, Veenhuyzen GD, Bordachar P, Chauvin M, Jais P, Coureau G, Chene G, Klein GJ & Clementy J. (2008) Sudden cardiac arrest associated with early repolarization. N Engl J Med 358(19): 2016–2023. Halkin A, Steinvil A, Rosso R, Adler A, Rozovski U & Viskin S. (2012) Preventing sudden death of athletes with electrocardiographic screening: what is the absolute benefit and how much will it cost? J Am Coll Cardiol 60(22): 2271–2276. Hall AS & Palmer S. (2008) The heart rate hypothesis: ready to be tested. Heart 94(5): 561–565. Hancock EW, Deal BJ, Mirvis DM, Okin P, Kligfield P, Gettes LS, Bailey JJ, Childers R, Gorgels A, Josephson M, Kors JA, Macfarlane P, Mason JW, Pahlm O, Rautaharju PM, Surawicz B, van Herpen G, Wagner GS, Wellens H, American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology, American College of Cardiology Foundation & Heart Rhythm Society. (2009) AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part V: electrocardiogram changes associated with cardiac chamber hypertrophy: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol 53(11): 992–1002. Harald K, Koskinen S, Jousilahti P, Torppa J, Vartiainen E & Salomaa V. (2008) Changes in traditional risk factors no longer explain time trends in cardiovascular mortality and its socioeconomic differences. J Epidemiol Community Health 62(3): 251–257. 78 Hiss RG, Averill KH & Lamb LE. (1960) Electrocardiographic findings in 67,375 asymptomatic subjects. VIII. Nonspecific T wave changes. Am J Cardiol 6: 178–189. Hiss RG & Lamb LE. (1962) Electrocardiographic findings in 122,043 individuals. Circulation 25: 947–961. Holm H, Gudbjartsson DF, Arnar DO, Thorleifsson G, Thorgeirsson G, Stefansdottir H, Gudjonsson SA, Jonasdottir A, Mathiesen EB, Njolstad I, Nyrnes A, Wilsgaard T, Hald EM, Hveem K, Stoltenberg C, Lochen ML, Kong A, Thorsteinsdottir U & Stefansson K. (2010) Several common variants modulate heart rate, PR interval and QRS duration. Nat Genet 42(2): 117–122. Horwich T, Lee SJ & Saxon L. (2003) Usefulness of QRS prolongation in predicting risk of inducible monomorphic ventricular tachycardia in patients referred for electrophysiologic studies. Am J Cardiol 92(7): 804–809. Hsieh BP, Pham MX & Froelicher VF. (2005) Prognostic value of electrocardiographic criteria for left ventricular hypertrophy. Am Heart J 150(1): 161–167. Huikuri HV, Castellanos A & Myerburg RJ. (2001) Sudden death due to cardiac arrhythmias. N Engl J Med 345(20): 1473–1482. Jain R, Dalal D, Daly A, Tichnell C, James C, Evenson A, Jain R, Abraham T, Tan BY, Tandri H, Russell SD, Judge D & Calkins H. (2009) Electrocardiographic features of arrhythmogenic right ventricular dysplasia. Circulation 120(6): 477–487. Josephson ME. (2008a) Atrioventricular Conduction. In: Anonymous Clinical Cardiac Electrophysiology: Techniques and Interpretation. Philadelphia: Lippincott Williams & Wilkins: 93–113. Josephson ME. (2008b) Recurrent Ventricular Tachycardia. In: Anonymous Clinical Cardiac Electrophysiology: Techniques and Interpretation. Philadelphia: Lippincott Williams & Wilkins: 446–642. Jouven X, Empana JP, Schwartz PJ, Desnos M, Courbon D & Ducimetiere P. (2005) Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med 352(19): 1951–1958. Junttila MJ, Sager SJ, Tikkanen JT, Anttonen O, Huikuri HV & Myerburg RJ. (2012) Clinical significance of variants of J-points and J-waves: early repolarization patterns and risk. Eur Heart J 33(21): 2639–2643. Kardys I, Kors JA, van der Meer IM, Hofman A, van der Kuip DA & Witteman JC. (2003) Spatial QRS-T angle predicts cardiac death in a general population. Eur Heart J 24(14): 1357–1364. Kashani A & Barold SS. (2005) Significance of QRS complex duration in patients with heart failure. J Am Coll Cardiol 46(12): 2183–2192. Kennedy HL, Whitlock JA, Sprague MK, Kennedy LJ, Buckingham TA & Goldberg RJ. (1985) Long-term follow-up of asymptomatic healthy subjects with frequent and complex ventricular ectopy. N Engl J Med 312(4): 193–197. Kistler PM, Sanders P, Fynn SP, Stevenson IH, Spence SJ, Vohra JK, Sparks PB & Kalman JM. (2004) Electrophysiologic and electroanatomic changes in the human atrium associated with age. J Am Coll Cardiol 44(1): 109–116. 79 Kjekshus J. (1990) Arrhythmias and mortality in congestive heart failure. Am J Cardiol 65(19): 42I-48I. Klatsky AL, Oehm R, Cooper RA, Udaltsova N & Armstrong MA. (2003) The early repolarization normal variant electrocardiogram: correlates and consequences. Am J Med 115(3): 171–177. Kligfield P. (2002) The centennial of the Einthoven electrocardiogram. J Electrocardiol 35 Suppl: 123–129. Kors JA, de Bruyne MC, Hoes AW, van Herpen G, Hofman A, van Bemmel JH & Grobbee DE. (1998) T axis as an indicator of risk of cardiac events in elderly people. Lancet 352(9128): 601–605. Kors JA, Kardys I, van der Meer IM, van Herpen G, Hofman A, van der Kuip DA & Witteman JC. (2003) Spatial QRS-T angle as a risk indicator of cardiac death in an elderly population. J Electrocardiol 36 Suppl: 113–114. Kurl S, Makikallio T, Rautaharju P, Kiviniemi V & Laukkanen JA. (2012) The Duration of QRS Complex in Resting Electrocardiogram is a Predictor of Sudden Cardiac Death in Men. Circulation 125(21): 2588–2594. Lahtinen AM, Lehtonen E, Marjamaa A, Kaartinen M, Helio T, Porthan K, Oikarinen L, Toivonen L, Swan H, Jula A, Peltonen L, Palotie A, Salomaa V & Kontula K. (2011) Population-prevalent desmosomal mutations predisposing to arrhythmogenic right ventricular cardiomyopathy. Heart Rhythm 8(8): 1214–1221. Lee SJ, McCulloch C, Mangat I, Foster E, De Marco T & Saxon LA. (2003) Isolated bundle branch block and left ventricular dysfunction. J Card Fail 9(2): 87–92. Lev M. (1964) Anatomic Basis for Atrioventricular Block. Am J Med 37: 742–748. Macfarlane PW. (2012) The frontal plane QRS-T angle. Europace 14(6): 773–775. Macfarlane PW & Lawrie TDV. (1989) Vol 3. In: Anonymous Comprehensive Electrocardiology. : Oxford: Pergamon Press: 1459. Magnani JW, Gorodeski EZ, Johnson VM, Sullivan LM, Hamburg NM, Benjamin EJ & Ellinor PT. (2011) P wave duration is associated with cardiovascular and all-cause mortality outcomes: the National Health and Nutrition Examination Survey. Heart Rhythm 8(1): 93–100. Magnani JW, Wang N, Nelson KP, Connelly S, Deo R, Rodondi N, Schelbert EB, Garcia ME, Phillips CL, Shlipak MG, Harris TB, Ellinor PT & Benjamin EJ. (2013) The Electrocardiographic PR Interval and Adverse Outcomes in Older Adults: the Health, Aging and Body Composition Study. Circ Arrhythm Electrophysiol 6(1): 84–90. Marcus FI. (2005) Prevalence of T-wave inversion beyond V1 in young normal individuals and usefulness for the diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia. Am J Cardiol 95(9): 1070–1071. Marcus FI, Fontaine GH, Guiraudon G, Frank R, Laurenceau JL, Malergue C & Grosgogeat Y. (1982) Right ventricular dysplasia: a report of 24 adult cases. Circulation 65(2): 384–398. 80 Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, Calkins H, Corrado D, Cox MG, Daubert JP, Fontaine G, Gear K, Hauer R, Nava A, Picard MH, Protonotarios N, Saffitz JE, Sanborn DM, Steinberg JS, Tandri H, Thiene G, Towbin JA, Tsatsopoulou A, Wichter T & Zareba W. (2010) Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation 121(13): 1533–1541. Marcus FI & Zareba W. (2009) The electrocardiogram in right ventricular cardiomyopathy/dysplasia. How can the electrocardiogram assist in understanding the pathologic and functional changes of the heart in this disease? J Electrocardiol 42(2): 136.e1–136.e5. Marcus FI, Zareba W, Calkins H, Towbin JA, Basso C, Bluemke DA, Estes NA,3rd, Picard MH, Sanborn D, Thiene G, Wichter T, Cannom D, Wilber DJ, Scheinman M, Duff H, Daubert J, Talajic M, Krahn A, Sweeney M, Garan H, Sakaguchi S, Lerman BB, Kerr C, Kron J, Steinberg JS, Sherrill D, Gear K, Brown M, Severski P, Polonsky S & McNitt S. (2009) Arrhythmogenic right ventricular cardiomyopathy/dysplasia clinical presentation and diagnostic evaluation: results from the North American Multidisciplinary Study. Heart Rhythm 6(7): 984–992. Massing MW, Simpson RJ,Jr, Rautaharju PM, Schreiner PJ, Crow R & Heiss G. (2006) Usefulness of ventricular premature complexes to predict coronary heart disease events and mortality (from the Atherosclerosis Risk In Communities cohort). Am J Cardiol 98(12): 1609–1612. Mazzoleni A, Curtin ME, Wolff R, Reiner L & Somes G. (1975) On the relationship between heart weights, fibrosis, and QRS duration. J Electrocardiol 8(3): 233–236. McKenna WJ, Thiene G, Nava A, Fontaliran F, Blomstrom-Lundqvist C, Fontaine G & Camerini F. (1994) Diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Task Force of the Working Group Myocardial and Pericardial Disease of the European Society of Cardiology and of the Scientific Council on Cardiomyopathies of the International Society and Federation of Cardiology. Br Heart J 71(3): 215–218. Migliore F, Zorzi A, Michieli P, Perazzolo Marra M, Siciliano M, Rigato I, Bauce B, Basso C, Toazza D, MSchiavon M, Iliceto S, Thiene G & Corrado D. (2012) Prevalence of Cardiomyopathy in Italian Asymptomatic Children With Electrocardiographic T-Wave Inversion at Preparticipation Screening. Circulation 125: 529–538. Miller WL, Ballman KV, Hodge DO, Rodeheffer RJ & Hammill SC. (2005) Risk factor implications of incidentally discovered uncomplicated bundle branch block. Mayo Clin Proc 80(12): 1585–1590. Mirvis DM & Goldberger AL. (2008) Electrocardiography. In: Libby P, Bonow RO, Mann DL & Zipes DP (eds) Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. Philadelphia, Pennsylvania: Saunders Elsevier: 149–193. Modi S & Krahn AD. (2011) Sudden cardiac arrest without overt heart disease. Circulation 123(25): 2994–3008. 81 Morin DP, Oikarinen L, Viitasalo M, Toivonen L, Nieminen MS, Kjeldsen SE, Dahlof B, John M, Devereux RB & Okin PM. (2009) QRS duration predicts sudden cardiac death in hypertensive patients undergoing intensive medical therapy: the LIFE study. Eur Heart J 30(23): 2908–2914. Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, Estes NA,3rd, Foster E, Greenberg H, Higgins SL, Pfeffer MA, Solomon SD, Wilber D, Zareba W & MADITCRT Trial Investigators. (2009) Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med 361(14): 1329–1338. Moyer VA & U.S. Preventive Services Task Force. (2012) Screening for coronary heart disease with electrocardiography: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 157(7): 512–518. Myerburg RJ & Junttila MJ. (2012) Sudden cardiac death caused by coronary heart disease. Circulation 125(8): 1043–1052. Myerburg RJ, Mitrani R, Interian A,Jr & Castellanos A. (1998) Interpretation of outcomes of antiarrhythmic clinical trials: design features and population impact. Circulation 97(15): 1514–1521. Mymin D, Mathewson FA, Tate RB & Manfreda J. (1986) The natural history of primary first-degree atrioventricular heart block. N Engl J Med 315(19): 1183–1187. Nam GB, Kim YH & Antzelevitch C. (2008) Augmentation of J waves and electrical storms in patients with early repolarization. N Engl J Med 358(19): 2078–2079. Napolitano C, Bloise R, Monteforte N & Priori SG. (2012) Sudden cardiac death and genetic ion channelopathies: long QT, Brugada, short QT, catecholaminergic polymorphic ventricular tachycardia, and idiopathic ventricular fibrillation. Circulation 125(16): 2027–2034. Nasir K, Bomma C, Tandri H, Roguin A, Dalal D, Prakasa K, Tichnell C, James C, Spevak PJ, Marcus F & Calkins H. (2004) Electrocardiographic features of arrhythmogenic right ventricular dysplasia/cardiomyopathy according to disease severity: a need to broaden diagnostic criteria. Circulation 110(12): 1527–1534. Nava A, Canciani B, Buja G, Martini B, Daliento L, Scognamiglio R & Thiene G. (1988) Electrovectorcardiographic study of negative T waves on precordial leads in arrhythmogenic right ventricular dysplasia: relationship with right ventricular volumes. J Electrocardiol 21(3): 239–245. Nerbonne JM & Kass RS. (2005) Molecular physiology of cardiac repolarization. Physiol Rev 85(4): 1205–1253. Newby KH, Pisano E, Krucoff MW, Green C & Natale A. (1996) Incidence and clinical relevance of the occurrence of bundle-branch block in patients treated with thrombolytic therapy. Circulation 94(10): 2424–2428. Newton-Cheh C, Guo CY, Wang TJ, O'donnell CJ, Levy D & Larson MG. (2007) Genome-wide association study of electrocardiographic and heart rate variability traits: the Framingham Heart Study. BMC Med Genet 8 Suppl 1: S7. 82 Nichol G, Thomas E, Callaway CW, Hedges J, Powell JL, Aufderheide TP, Rea T, Lowe R, Brown T, Dreyer J, Davis D, Idris A, Stiell I & Resuscitation Outcomes Consortium Investigators. (2008) Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA 300(12): 1423–1431. Niwano S, Wakisaka Y, Niwano H, Fukaya H, Kurokawa S, Kiryu M, Hatakeyama Y & Izumi T. (2009) Prognostic significance of frequent premature ventricular contractions originating from the ventricular outflow tract in patients with normal left ventricular function. Heart 95(15): 1230–1237. Oikarinen L, Nieminen MS, Viitasalo M, Toivonen L, Jern S, Dahlof B, Devereux RB, Okin PM & LIFE Study Investigators. (2004) QRS duration and QT interval predict mortality in hypertensive patients with left ventricular hypertrophy: the Losartan Intervention for Endpoint Reduction in Hypertension Study. Hypertension 43(5): 1029–1034. Okada M, Yotsukura M, Shimada T & Ishikawa K. (1994) Clinical implications of isolated T wave inversion in adults: electrocardiographic differentiation of the underlying causes of this phenomenon. J Am Coll Cardiol 24(3): 739–745. Okin PM. (2006) Electrocardiography in women: taking the initiative. Circulation 113(4): 464–466. O'Malley PG & Redberg RF. (2010) Risk refinement, reclassification, and treatment thresholds in primary prevention of cardiovascular disease: incremental progress but significant gaps remain. Arch Intern Med 170(17): 1602–1603. Packard JM, Graettinger JS & Graybiel A. (1954) Analysis of the electrocardiograms obtained from 1000 young healthy aviators; ten year follow-up. Circulation 10(3): 384–400. Pajunen P, Koukkunen H, Ketonen M, Jerkkola T, Immonen-Raiha P, Karja-Koskenkari P, Mahonen M, Niemela M, Kuulasmaa K, Palomaki P, Mustonen J, Lehtonen A, Arstila M, Vuorenmaa T, Lehto S, Miettinen H, Torppa J, Tuomilehto J, Kesaniemi YA, Pyorala K & Salomaa V. (2005) The validity of the Finnish Hospital Discharge Register and Causes of Death Register data on coronary heart disease. Eur J Cardiovasc Prev Rehabil 12(2): 132–137. Papadakis M, Basavarajaiah S, Rawlins J, Edwards C, Makan J, Firoozi S, Carby L & Sharma S. (2009) Prevalence and significance of T-wave inversions in predominantly Caucasian adolescent athletes. Eur Heart J 30(14): 1728–1735. Pappano AJ. (2008) Elements of Cardiac Function. In: Koeppen BM & Stanton BA (eds) Berne and Levy Physiology. Philadelphia, Pennsylvania: Mosby, Elsevier: 292–329. Pavri BB, Hillis MB, Subacius H, Brumberg GE, Schaechter A, Levine JH, Kadish A & Defibrillators in Nonischemic Cardiomyopathy Treatment Evaluation (DEFINITE) Investigators. (2008) Prognostic value and temporal behavior of the planar QRS-T angle in patients with nonischemic cardiomyopathy. Circulation 117(25): 3181–3186. 83 Pelliccia A, Culasso F, Di Paolo FM, Accettura D, Cantore R, Castagna W, Ciacciarelli A, Costini G, Cuffari B, Drago E, Federici V, Gribaudo CG, Iacovelli G, Landolfi L, Menichetti G, Atzeni UO, Parisi A, Pizzi AR, Rosa M, Santelli F, Santilio F, Vagnini A, Casasco M & Di Luigi L. (2007) Prevalence of abnormal electrocardiograms in a large, unselected population undergoing pre-participation cardiovascular screening. Eur Heart J 28(16): 2006–2010. Pelliccia A, Di Paolo FM, Quattrini FM, Basso C, Culasso F, Popoli G, De Luca R, Spataro A, Biffi A, Thiene G & Maron BJ. (2008) Outcomes in athletes with marked ECG repolarization abnormalities. N Engl J Med 358(2): 152–161. Pelliccia A, Maron BJ, Culasso F, Di Paolo FM, Spataro A, Biffi A, Caselli G & Piovano P. (2000) Clinical significance of abnormal electrocardiographic patterns in trained athletes. Circulation 102(3): 278–284. Perlman LV, Ostrander LD,Jr, Keller JB & Chiang BN. (1971) An epidemiologic study of first degree atrioventricular block in Tecumseh, Michigan. Chest 59(1): 40–46. Peters S, Trummel M & Meyners W. (2004) Prevalence of right ventricular dysplasiacardiomyopathy in a non-referral hospital. Int J Cardiol 97(3): 499–501. Pfeufer A, van Noord C, Marciante KD, Arking DE, Larson MG, Smith AV, Tarasov KV, Muller M, Sotoodehnia N, Sinner MF, Verwoert GC, Li M, Kao WH, Kottgen A, Coresh J, Bis JC, Psaty BM, Rice K, Rotter JI, Rivadeneira F, Hofman A, Kors JA, Stricker BH, Uitterlinden AG, van Duijn CM, Beckmann BM, Sauter W, Gieger C, Lubitz SA, Newton-Cheh C, Wang TJ, Magnani JW, Schnabel RB, Chung MK, Barnard J, Smith JD, Van Wagoner DR, Vasan RS, Aspelund T, Eiriksdottir G, Harris TB, Launer LJ, Najjar SS, Lakatta E, Schlessinger D, Uda M, Abecasis GR, MullerMyhsok B, Ehret GB, Boerwinkle E, Chakravarti A, Soliman EZ, Lunetta KL, Perz S, Wichmann HE, Meitinger T, Levy D, Gudnason V, Ellinor PT, Sanna S, Kaab S, Witteman JC, Alonso A, Benjamin EJ & Heckbert SR. (2010) Genome-wide association study of PR interval. Nat Genet 42(2): 153–159. Pratt CM, Greenway PS, Schoenfeld MH, Hibben ML & Reiffel JA. (1996) Exploration of the precision of classifying sudden cardiac death. Implications for the interpretation of clinical trials. Circulation 93(3): 519–524. Prineas RJ, Crow RS & Blackburn H. (1982) The Minnesota Code Manual of Electrocardiographic Findings. Boston, Massachusetts: John Wright PSB. Prystowsky EN, Padanilam BJ, Joshi S & Fogel RI. (2012) Ventricular arrhythmias in the absence of structural heart disease. J Am Coll Cardiol 59(20): 1733–1744. Rabkin SW, Mathewson FA & Tate RB. (1980) Natural history of left bundle-branch block. Br Heart J 43(2): 164–169. Rampazzo A, Nava A, Danieli GA, Buja G, Daliento L, Fasoli G, Scognamiglio R, Corrado D & Thiene G. (1994) The gene for arrhythmogenic right ventricular cardiomyopathy maps to chromosome 14q23-q24. Hum Mol Genet 3(6): 959–962. Rapola JM, Virtamo J, Korhonen P, Haapakoski J, Hartman AM, Edwards BK & Heinonen OP. (1997) Validity of diagnoses of major coronary events in national registers of hospital diagnoses and deaths in Finland. Eur J Epidemiol 13(2): 133–138. 84 Rautaharju PM, Kooperberg C, Larson JC & LaCroix A. (2006a) Electrocardiographic abnormalities that predict coronary heart disease events and mortality in postmenopausal women: the Women's Health Initiative. Circulation 113(4): 473–480. Rautaharju PM, Kooperberg C, Larson JC & LaCroix A. (2006b) Electrocardiographic predictors of incident congestive heart failure and all-cause mortality in postmenopausal women: the Women's Health Initiative. Circulation 113(4): 481–489. Rautaharju PM, Nelson JC, Kronmal RA, Zhang ZM, Robbins J, Gottdiener JS, Furberg CD, Manolio T & Fried L. (2001) Usefulness of T-axis deviation as an independent risk indicator for incident cardiac events in older men and women free from coronary heart disease (the Cardiovascular Health Study). Am J Cardiol 88(2): 118–123. Rautaharju PM, Prineas RJ, Wood J, Zhang ZM, Crow R & Heiss G. (2007) Electrocardiographic predictors of new-onset heart failure in men and in women free of coronary heart disease (from the Atherosclerosis in Communities [ARIC] Study). Am J Cardiol 100(9): 1437–1441. Rautaharju PM, Surawicz B, Gettes LS, Bailey JJ, Childers R, Deal BJ, Gorgels A, Hancock EW, Josephson M, Kligfield P, Kors JA, Macfarlane P, Mason JW, Mirvis DM, Okin P, Pahlm O, van Herpen G, Wagner GS, Wellens H, American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology, American College of Cardiology Foundation & Heart Rhythm Society. (2009) AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV: the ST segment, T and U waves, and the QT interval: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol 53(11): 982–991. Reunanen A, Aromaa A, Pyorala K, Punsar S, Maatela J & Knekt P. (1983) The Social Insurance Institution's coronary heart disease study. Baseline data and 5-year mortality experience. Acta Med Scand Suppl 673: 1–120. Rose G, Baxter PJ, Reid DD & McCartney P. (1978) Prevalence and prognosis of electrocardiographic findings in middle-aged men. Br Heart J 40(6): 636–643. Rose GA. (1962) The diagnosis of ischaemic heart pain and intermittent claudication in field surveys. Bull World Health Organ 27: 645–658. Rubart M & Zipes DP. (2008) Genesis of Cardiac Arrhythmias: Electrophysiological Considerations. In: Libby P, Bonow RO, Mann DL & Zipes DP (eds) Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. Philadelphia, Pennsylvania: Saunders, Elsevier: 727–762. Sandhu R & Bahler RC. (2004) Prevalence of QRS prolongation in a community hospital cohort of patients with heart failure and its relation to left ventricular systolic dysfunction. Am J Cardiol 93(2): 244–246. 85 Schinkel AF, Elhendy A, van Domburg RT, Biagini E, Rizzello V, Veltman CE, Ten Kate GL, Sijbrands EJ, Akkerhuis KM, Geleijnse ML, Ten Cate FJ, Simoons ML, Bax JJ & Poldermans D. (2009) Prognostic significance of QRS duration in patients with suspected coronary artery disease referred for noninvasive evaluation of myocardial ischemia. Am J Cardiol 104(11): 1490–1493. Schneider JF, Thomas HE,Jr, Sorlie P, Kreger BE, McNamara PM & Kannel WB. (1981) Comparative features of newly acquired left and right bundle branch block in the general population: the Framingham study. Am J Cardiol 47(4): 931–940. Schnittger I, Appleton CP, Hatle LK & Popp RL. (1988) Diastolic mitral and tricuspid regurgitation by Doppler echocardiography in patients with atrioventricular block: new insight into the mechanism of atrioventricular valve closure. J Am Coll Cardiol 11(1): 83–88. Schwartz PJ, Stramba-Badiale M, Crotti L, Pedrazzini M, Besana A, Bosi G, Gabbarini F, Goulene K, Insolia R, Mannarino S, Mosca F, Nespoli L, Rimini A, Rosati E, Salice P & Spazzolini C. (2009) Prevalence of the congenital long-QT syndrome. Circulation 120(18): 1761–1767. Schwartzman D. (2004) Atrioventricular Block and Atrioventricular Dissociation. In: Zipes DP & Jalife J (eds) Cardiac Electrophysiology: From Cell to Bedside. Philadelphia, Pennsylvania: Saunders/Elsevier: 485–489. Sen-Chowdhry S & McKenna WJ. (2012) Sudden death from genetic and acquired cardiomyopathies. Circulation 125(12): 1563–1576. Sigurdsson E, Sigfusson N, Sigvaldason H & Thorgeirsson G. (1996) Silent ST-T changes in an epidemiologic cohort study--a marker of hypertension or coronary heart disease, or both: the Reykjavik study. J Am Coll Cardiol 27(5): 1140–1147. Smith JG, Lowe JK, Kovvali S, Maller JB, Salit J, Daly MJ, Stoffel M, Altshuler DM, Friedman JM, Breslow JL & Newton-Cheh C. (2009) Genome-wide association study of electrocardiographic conduction measures in an isolated founder population: Kosrae. Heart Rhythm 6(5): 634–641. Soliman EZ, Prineas RJ, Case LD, Zhang ZM & Goff DC,Jr. (2009) Ethnic distribution of ECG predictors of atrial fibrillation and its impact on understanding the ethnic distribution of ischemic stroke in the Atherosclerosis Risk in Communities (ARIC) study. Stroke 40(4): 1204–1211. 86 Sotoodehnia N, Isaacs A, de Bakker PI, Dorr M, Newton-Cheh C, Nolte IM, van der Harst P, Muller M, Eijgelsheim M, Alonso A, Hicks AA, Padmanabhan S, Hayward C, Smith AV, Polasek O, Giovannone S, Fu J, Magnani JW, Marciante KD, Pfeufer A, Gharib SA, Teumer A, Li M, Bis JC, Rivadeneira F, Aspelund T, Kottgen A, Johnson T, Rice K, Sie MP, Wang YA, Klopp N, Fuchsberger C, Wild SH, Mateo Leach I, Estrada K, Volker U, Wright AF, Asselbergs FW, Qu J, Chakravarti A, Sinner MF, Kors JA, Petersmann A, Harris TB, Soliman EZ, Munroe PB, Psaty BM, Oostra BA, Cupples LA, Perz S, de Boer RA, Uitterlinden AG, Volzke H, Spector TD, Liu FY, Boerwinkle E, Dominiczak AF, Rotter JI, van Herpen G, Levy D, Wichmann HE, van Gilst WH, Witteman JC, Kroemer HK, Kao WH, Heckbert SR, Meitinger T, Hofman A, Campbell H, Folsom AR, van Veldhuisen DJ, Schwienbacher C, O'Donnell CJ, Volpato CB, Caulfield MJ, Connell JM, Launer L, Lu X, Franke L, Fehrmann RS, te Meerman G, Groen HJ, Weersma RK, van den Berg LH, Wijmenga C, Ophoff RA, Navis G, Rudan I, Snieder H, Wilson JF, Pramstaller PP, Siscovick DS, Wang TJ, Gudnason V, van Duijn CM, Felix SB, Fishman GI, Jamshidi Y, Stricker BH, Samani NJ, Kaab S & Arking DE. (2010) Common variants in 22 loci are associated with QRS duration and cardiac ventricular conduction. Nat Genet 42(12): 1068–1076. Stecker EC, Vickers C, Waltz J, Socoteanu C, John BT, Mariani R, McAnulty JH, Gunson K, Jui J & Chugh SS. (2006) Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: two-year findings from the Oregon Sudden Unexpected Death Study. J Am Coll Cardiol 47(6): 1161–1166. Stein KM. (2008) Noninvasive risk stratification for sudden death: signal-averaged electrocardiography, nonsustained ventricular tachycardia, heart rate variability, baroreflex sensitivity, and QRS duration. Prog Cardiovasc Dis 51(2): 106–117. Steinvil A, Chundadze T, Zeltser D, Rogowski O, Halkin A, Galily Y, Perluk H & Viskin S. (2011) Mandatory electrocardiographic screening of athletes to reduce their risk for sudden death proven fact or wishful thinking? J Am Coll Cardiol 57(11): 1291–1296. Stevenson WG, Stevenson LW, Middlekauff HR & Saxon LA. (1993) Sudden death prevention in patients with advanced ventricular dysfunction. Circulation 88(6): 2953– 2961. Straus SM, Kors JA, De Bruin ML, van der Hooft CS, Hofman A, Heeringa J, Deckers JW, Kingma JH, Sturkenboom MC, Stricker BH & Witteman JC. (2006) Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol 47(2): 362–367. Suarez RM & Suarez RM,Jr. (1946) The T-wave of the precordial electrocardiogram at different age levels. Am Heart J 32(4): 480–493. Sullivan JM, Vander Zwaag RV, el-Zeky F, Ramanathan KB & Mirvis DM. (1993) Left ventricular hypertrophy: effect on survival. J Am Coll Cardiol 22(2): 508–513. Sumner G, Salehian O, Yi Q, Healey J, Mathew J, Al-Merri K, Al-Nemer K, Mann JF, Dagenais G, Lonn E & HOPE Investigators. (2009) The prognostic significance of bundle branch block in high-risk chronic stable vascular disease patients: a report from the HOPE trial. J Cardiovasc Electrophysiol 20(7): 781–787. 87 Surawicz B, Childers R, Deal BJ, Gettes LS, Bailey JJ, Gorgels A, Hancock EW, Josephson M, Kligfield P, Kors JA, Macfarlane P, Mason JW, Mirvis DM, Okin P, Pahlm O, Rautaharju PM, van Herpen G, Wagner GS, Wellens H, American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology, American College of Cardiology Foundation & Heart Rhythm Society. (2009) AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part III: intraventricular conduction disturbances: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol 53(11): 976–981. Swedberg K, Komajda M, Bohm M, Borer JS, Ford I, Dubost-Brama A, Lerebours G, Tavazzi L & SHIFT Investigators. (2010) Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet 376(9744): 875–885. Taneja T, Mahnert BW, Passman R, Goldberger J & Kadish A. (2001) Effects of sex and age on electrocardiographic and cardiac electrophysiological properties in adults. Pacing Clin Electrophysiol 24(1): 16–21. Teodorescu C, Reinier K, Uy-Evanado A, Navarro J, Mariani R, Gunson K, Jui J & Chugh SS. (2011) Prolonged QRS duration on the resting ECG is associated with sudden death risk in coronary disease, independent of prolonged ventricular repolarization. Heart Rhythm 8(10): 1562–1567. Thiene G, Nava A, Corrado D, Rossi L & Pennelli N. (1988) Right ventricular cardiomyopathy and sudden death in young people. N Engl J Med 318(3): 129–133. Thompson PD, Franklin BA, Balady GJ, Blair SN, Corrado D, Estes NA,3rd, Fulton JE, Gordon NF, Haskell WL, Link MS, Maron BJ, Mittleman MA, Pelliccia A, Wenger NK, Willich SN, Costa F, American Heart Association Council on Nutrition, Physical Activity, and Metabolism, American Heart Association Council on Clinical Cardiology & American College of Sports Medicine. (2007) Exercise and acute cardiovascular events placing the risks into perspective: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism and the Council on Clinical Cardiology. Circulation 115(17): 2358–2368. Thrainsdottir IS, Hardarson T, Thorgeirsson G, Sigvaldason H & Sigfusson N. (1993) The epidemiology of right bundle branch block and its association with cardiovascular morbidity--the Reykjavik Study. Eur Heart J 14(12): 1590–1596. Tikkanen JT, Anttonen O, Junttila MJ, Aro AL, Kerola T, Rissanen HA, Reunanen A & Huikuri HV. (2009) Long-term outcome associated with early repolarization on electrocardiography. N Engl J Med 361(26): 2529–2537. Tikkanen JT, Junttila MJ, Anttonen O, Aro AL, Luttinen S, Kerola T, Sager SJ, Rissanen HA, Myerburg RJ, Reunanen A & Huikuri HV. (2011) Early repolarization: electrocardiographic phenotypes associated with favorable long-term outcome. Circulation 123(23): 2666–2673. Toivonen L, Helenius K & Viitasalo M. (1997) Electrocardiographic repolarization during stress from awakening on alarm call. J Am Coll Cardiol 30(3): 774–779. 88 Uberoi A, Jain NA, Perez M, Weinkopff A, Ashley E, Hadley D, Turakhia MP & Froelicher V. (2011) Early repolarization in an ambulatory clinical population. Circulation 124(20): 2208–2214. Uhm JS, Hwang IU, Oh YS, Choi MS, Jang SW, Shin WS, Kim JH, Lee MY, Rho TH, Kim YH, Sung JH, Lee YS, Cho JG, Oh DJ, Kim DK, Namgung J, Park KM, Kim YH, Kim YN, Lim HE, Cha TJ, On YK, Shin DG, Pak HN & Kim NH. (2011) Prevalence of electrocardiographic findings suggestive of sudden cardiac death risk in 10,867 apparently healthy young Korean men. Pacing Clin Electrophysiol 34(6): 717– 723. Villuendas R & Kadish AH. (2008) Cardiac magnetic resonance for risk stratification: the sudden death risk portrayed. Prog Cardiovasc Dis 51(2): 128–134. Wang NC, Maggioni AP, Konstam MA, Zannad F, Krasa HB, Burnett JC,Jr, Grinfeld L, Swedberg K, Udelson JE, Cook T, Traver B, Zimmer C, Orlandi C, Gheorghiade M & Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) Investigators. (2008) Clinical implications of QRS duration in patients hospitalized with worsening heart failure and reduced left ventricular ejection fraction. JAMA 299(22): 2656–2666. Wilson MG, Sharma S, Carre F, Charron P, Richard P, O'Hanlon R, Prasad SK, Heidbuchel H, Brugada J, Salah O, Sheppard M, George KP, Whyte G, Hamilton B & Chalabi H. (2012) Significance of deep T-wave inversions in asymptomatic athletes with normal cardiovascular examinations: practical solutions for managing the diagnostic conundrum. Br J Sports Med 46 Suppl 1: i51–8. Yamazaki T, Froelicher VF, Myers J, Chun S & Wang P. (2005) Spatial QRS-T angle predicts cardiac death in a clinical population. Heart Rhythm 2(1): 73–78. Yan GX, Lankipalli RS, Burke JF, Musco S & Kowey PR. (2003) Ventricular repolarization components on the electrocardiogram: cellular basis and clinical significance. J Am Coll Cardiol 42(3): 401–409. Yan GX, Shimizu W & Antzelevitch C. (1998) Characteristics and distribution of M cells in arterially perfused canine left ventricular wedge preparations. Circulation 98(18): 1921–1927. Young LH, Wackers FJ, Chyun DA, Davey JA, Barrett EJ, Taillefer R, Heller GV, Iskandrian AE, Wittlin SD, Filipchuk N, Ratner RE, Inzucchi SE & DIAD Investigators. (2009) Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA 301(15): 1547–1555. Zabel M, Acar B, Klingenheben T, Franz MR, Hohnloser SH & Malik M. (2000) Analysis of 12-lead T-wave morphology for risk stratification after myocardial infarction. Circulation 102(11): 1252–1257. Zhang Y, Post WS, Dalal D, Blasco-Colmenares E, Tomaselli GF & Guallar E. (2011) QTinterval duration and mortality rate: results from the Third National Health and Nutrition Examination Survey. Arch Intern Med 171(19): 1727–1733. 89 Zhang ZM, Prineas RJ, Case D, Soliman EZ, Rautaharju PM & ARIC Research Group. (2007) Comparison of the prognostic significance of the electrocardiographic QRS/T angles in predicting incident coronary heart disease and total mortality (from the atherosclerosis risk in communities study). Am J Cardiol 100(5): 844–849. Zheng ZJ, Croft JB, Giles WH & Mensah GA. (2001) Sudden cardiac death in the United States, 1989 to 1998. Circulation 104(18): 2158–2163. Zimetbaum PJ, Buxton AE, Batsford W, Fisher JD, Hafley GE, Lee KL, O'Toole MF, Page RL, Reynolds M & Josephson ME. (2004) Electrocardiographic predictors of arrhythmic death and total mortality in the multicenter unsustained tachycardia trial. Circulation 110(7): 766–769. Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, Gregoratos G, Klein G, Moss AJ, Myerburg RJ, Priori SG, Quinones MA, Roden DM, Silka MJ, Tracy C, Smith SC,Jr, Jacobs AK, Adams CD, Antman EM, Anderson JL, Hunt SA, Halperin JL, Nishimura R, Ornato JP, Page RL, Riegel B, Blanc JJ, Budaj A, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL, American College of Cardiology/American Heart Association Task Force, European Society of Cardiology Committee for Practice Guidelines, European Heart Rhythm Association & Heart Rhythm Society. (2006) ACC/AHA/ESC 2006 Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (writing committee to develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation 114(10): e385–484. 90 Original publications I Aro AL, Anttonen O, Tikkanen JT, Junttila MJ, Kerola T, Rissanen HA, Reunanen A & Huikuri HV (2011) Intraventricular conduction delay in a standard 12-lead electrocardiogram as a predictor of mortality in the general population. Circ Arrhythm Electrophysiol 4(5): 704–710. II Aro AL, Huikuri HV, Tikkanen JT, Junttila MJ, Rissanen HA, Reunanen A & Anttonen O (2012) QRS-T angle as a predictor of sudden cardiac death in a middleaged general population. Europace 14(6): 872–876. III Aro AL, Anttonen O, Tikkanen JT, Junttila MJ, Kerola T, Rissanen HA, Reunanen A & Huikuri HV (2012) Prevalence and prognostic significance of T-wave inversions in right precordial leads of a 12-lead electrocardiogram in the middle-aged subjects. Circulation 125(21): 2572–2577. IV Aro AL, Anttonen O, Kerola T, Junttila MJ, Tikkanen JT, Rissanen HA, Reunanen A & Huikuri HV (2013) Prognostic significance of prolonged PR interval in the general population. European Heart Journal [Epub ahead of print May 14, 2013]. Reprinted with permission from Wolters Kluwer Health (I & III) and Oxford University Press (II & IV). Original publications are not included in the electronic version of the dissertation. 91 92 ACTA UNIVERSITATIS OULUENSIS SERIES D MEDICA 1195. Meriläinen, Sanna (2013) Experimental study of acute pancreatitis in a porcine model, especially tight junction structure and portal vein cytokines 1196. Koivikko, Minna (2013) Cardiac autonomic regulation and repolarisation during hypoglycaemia in type 1 diabetes 1197. Aro, Ellinoora (2013) Prolyl 4-hydroxylases, key enzymes regulating hypoxia response and collagen synthesis : the roles of specific isoenzymes in the control of erythropoiesis and skeletogenesis 1198. Koivumäki, Janne (2013) Biomechanical modeling of proximal femur : development of finite element models to simulate fractures 1199. Paavola, Liisa (2013) Salla disease – rare but diverse : a clinical follow-up study of a Finnish patient sample 1200. Vesterinen, Soili (2013) Osastonhoitajien johtamistyylit osana johtamiskulttuuria 1201. Huusko, Tuija (2013) Genetic and molecular background of ascending aortic aneurysms 1202. Pisto, Pauliina (2013) Fat accumulation in liver and muscle : association with adipokines and risk of cardiovascular events 1203. Yannopoulos, Fredrik (2013) Remote ischemic preconditioning as a means to protect the brain against hypothermic circulatory arrest : an experimental study on piglets 1204. Arvonen, Miika (2013) Intestinal immune activation in Juvenile idiopathic arthritis 1205. Ahonkallio, Sari (2013) Endometrial thermal ablation : a choice for treatment of heavy menstrual bleeding 1206. Penttilä, Matti (2013) Duration of untreated psychosis : association with clinical and social outcomes and brain morphology in schizophrenia 1207. Wikström, Erika (2013) Epidemiology of Chlamydia trachomatis infection in Finland during 1983–2009 1208. Jokela, Tiina (2013) Analyses of kidney organogenesis through in vitro and in vivo approaches : generation of conditional Wnt4 mouse models and a method for applying inducible Cre-recombination for kidney organ culture 1209. Hietikko, Elina (2013) Genetic and clinical features of familial Meniere’s disease in Northern Ostrobothnia and Kainuu 1210. Abou Elseoud, Ahmed (2013) Exploring functional brain networks using independent component analysis : functional brain networks connectivity Book orders: Granum: Virtual book store http://granum.uta.fi/granum/ D 1211 OULU 2013 UNIV ER S IT Y OF OULU P. O. B R[ 00 FI-90014 UNIVERSITY OF OULU FINLAND U N I V E R S I TAT I S S E R I E S SCIENTIAE RERUM NATURALIUM Senior Assistant Jorma Arhippainen HUMANIORA University Lecturer Santeri Palviainen TECHNICA Docent Hannu Heusala ACTA U N I V E R S I T AT I S O U L U E N S I S Aapo Aro E D I T O R S Aapo Aro A B C D E F G O U L U E N S I S ACTA A C TA D 1211 ELECTROCARDIOGRAPHIC RISK MARKERS OF SUDDEN CARDIAC DEATH IN MIDDLEAGED SUBJECTS MEDICA Professor Olli Vuolteenaho SCIENTIAE RERUM SOCIALIUM University Lecturer Hannu Heikkinen SCRIPTA ACADEMICA Director Sinikka Eskelinen OECONOMICA Professor Jari Juga EDITOR IN CHIEF Professor Olli Vuolteenaho PUBLICATIONS EDITOR Publications Editor Kirsti Nurkkala ISBN 978-952-62-0178-8 (Paperback) ISBN 978-952-62-0179-5 (PDF) ISSN 0355-3221 (Print) ISSN 1796-2234 (Online) UNIVERSITY OF OULU GRADUATE SCHOOL; UNIVERSITY OF OULU, FACULTY OF MEDICINE, INSTITUTE OF CLINICAL MEDICINE; OULU UNIVERSITY HOSPITAL; HELSINKI UNIVERSITY CENTRAL HOSPITAL, HEART AND LUNG CENTER D MEDICA