* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Double-slit experiment wikipedia , lookup
Orchestrated objective reduction wikipedia , lookup
Spin (physics) wikipedia , lookup
Coupled cluster wikipedia , lookup
Probability amplitude wikipedia , lookup
Coherent states wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
Particle in a box wikipedia , lookup
Franck–Condon principle wikipedia , lookup
Renormalization wikipedia , lookup
Quantum state wikipedia , lookup
Hartree–Fock method wikipedia , lookup
EPR paradox wikipedia , lookup
Interpretations of quantum mechanics wikipedia , lookup
Bohr–Einstein debates wikipedia , lookup
Scalar field theory wikipedia , lookup
Copenhagen interpretation wikipedia , lookup
Matter wave wikipedia , lookup
History of quantum field theory wikipedia , lookup
Renormalization group wikipedia , lookup
Canonical quantization wikipedia , lookup
Hidden variable theory wikipedia , lookup
Wave–particle duality wikipedia , lookup
Symmetry in quantum mechanics wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Molecular Hamiltonian wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
Chemical bond wikipedia , lookup
Tight binding wikipedia , lookup
Atomic theory wikipedia , lookup
Atomic orbital wikipedia , lookup
Hydrogen atom wikipedia , lookup
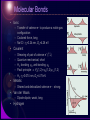
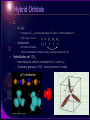
Physical Background Atomic and Molecular States • Rudiments of Quantum Theory – the old quantum theory – mathematical apparatus – interpretation • Atomic States – hydrogen – many-electron • Molecular states – classification – chemistry Rudiments of Quantum Theory Photons and Particles • Electromagnetic field – The wave equation – Plane waves, The wave function Maxwell equations Space and time change of phase 2 T 2 k c [rad / s] k 2 [rad / m] • Planck’s hypothesis, dualism – Particle (E,p)(,k) de Broglie wave Relativistic relation Ep E 2 c 2 p 2 m02 c 2 photon : p E c Bohr’s Model of the Hydrogen Atom • Classical atom – Unstable: accelerated motion, continuous radiation • Bohr’s rules – Quantized angular momentum • Only certain circular orbits allowed • Discrete set of stationary states • Discrete spectrum of energy – Discrete spectrum of radiation – Coulomb and centrifugal force – Bohr radius Mathematical Apparatus • Philosophy: Act of observation – Interaction through which the quantity is ‘observed’ General operator Aˆ Aˆ x, x – Possible results of observation – Non-commuting observations • Mathematics: operators in Hilbert space – Eigenvalue equation x x a1 0 0 a 2 Operator algebra x 1 x x x – Commutation relations x, x 1 Mathematical Apparatus • Interpretation postulates – Possible results of observation  are eigenvalues an – Observation  on a system in eigenstate n certainly leads to an – The mean value of the observable  on the ensemble of systems • Physical postulates – The correspondence principle • In the limit of ‘large’ system quantum laws reduce to classical laws • Relation between classical quantities with no derivatives holds also for quantum operators – The principle of complementarity • The Heisenberg uncertainty principle Mean value a * ( x) Aˆ ( x)dx * ( x) ( x)dx Complementarity principle perturbati on xˆ , pˆ x , x i u p exp ipx / gen.coordinate, gen.momentum Xˆ , Pˆ i Schrödinger representation “An experiment on one aspect of a system is supposed to destroy the possibility of learning about a 'complementary' aspect of the same system”. xˆ x; pˆ i x Angular Momentum • Space orientation of the orbit – Magnetic and electric moments • Internal and external interactions • Classical • Quantum – Spherical coordinates • Boundary condition +2n L mrv mr 2 I Ek p 2 2m 1 2 I 2 L2 2 I The Copenhagen Interpretation • radioactive isotope Probabilistic approach cyanide capsule – Probability density • Collapse of the wave function – Schrödinger’s Cat – Two-Slit Experiment Indeterminate quantum states “collapse” to definite values when they do, not when a human being catches them in the act Atomic States Hydrogen • • Particle in a central potential Coulomb potential [ Hˆ , lˆ 2 ] [ Hˆ , lˆz ] 0 n,l ,m SO coupling – internal Zeeman VB μB B – Electron spin – Fine structure: relativistic corrections • Electron-nucleus, Kinetic energy, Spinorbit interaction – Lamb shift – Hyperfine structure 0e l 4me r 3 μ e s me Hˆ SO (r )ˆlsˆ Hydrogen Many-Electron Atomic States • Ground state configuration – Pauli exclusion principle – Hund’s rules • e– with parallel s more separated – Lower repulsion, lower energy • Terms – LS coupling: small Z • L, S, J (M) – j-j coupling: large Z • J, M Molecular States Molecular Bonds • Ionic – Transfer of valence e– to produce a noble gas configuration – Coulomb force, long – Na+Cl-: re=0.24 nm, De=4.26 eV • Covalent – – – – – Shearing of pair of valence e– () Quantum mechanical, short H2: bonding S, anti-bonding A Pauli principle A(1,2)=S(1,2)(1,2) H2: re=0.074 nm, De=4.75 eV • Metallic – Shared and delocalized valence e– - strong • Van der Waals – Dipole-dipole, weak, long • Hydrogen Electronic States • Born-Oppenheimer Approximation U – Separation of electronic and nuclear motion U – Electronic motion – nuclei fixed internuclear distance r Electronic States • Classification – Total orbital momentum along internuclear axis in the electric field • Internal Stark effect – Total spin along internuclear axis • magnetic coupling – Parity of el • Inversion about a plane through the axis: +/• Inversion through the center of symmetry: g/u – Homonuclear molecules Electronic potentials Mg2 Nuclear Motion • Rigid rotator 2 ˆ2 J J ( J 1 ) Hˆ EJ 2I 2r 2 • Harmonic oscillator Harmonic (Hook) V(r) d 2 2 E V (r ) ; F kr; dr 2 2 1 1 V (r ) k (r re ) 2 cl2 (r re ) 2 ; 2 2 Ev cl (v 1 2) • Anharmonic oscillator – Morse potential V (r ) De 1 exp (r re ) Morse re De Nuclear Motion • Vibrating rotator – 100 vibrations during a revolution 1 – Averaged rotational constant Bv 2 r v – Mean value (1/r2)v decreases as v increases Bv Be e (v 1 2) – Centrifugal force • Coupling of electronic and nuclear motion – Hund’s cases • Coupling between various angular momentum vectors – Gyroscopic forces disturb orbital motion of electrons – Internal magnetic fields from the rotation of nuclei couple with the electron spin – Total angular momentum J Molecular Orbitals • • LCAO (Linear Combination of Atomic Orbitals), perturbation theory Homonuclear diatomics – Correlation diagram; surfaces of probability ||2, ||2 H2 united atoms He separated atoms H 3d 4d 4p 4s s 3p bonding + s antibonding 3s 2pg* 2pg 3p 3s 2p 2su* 2pu pz 2s + pz 2s 1su*` 1s 1s + internuclear distance re Li2 N2 + + bonding + + pzg pz + px + + + + pzu* pz bonding + + px px 1sg H2 + antibonding 2sg 2p + sg s u* s s * 2pu + + + + 3d energy + + + pxu antibonding + + px pxg* Hybrid Orbitals • C – 2s2pz • Increase of Esp less than decrease of E due to 4 bonds instead of 2 • CH4: 3sp + 1ss ? 1s 2s 2p 2p 2p – Hybridization x y z • All bonds the same • Linear combination of atomic s and p orbitals in case of EsEp • Hybridization sp3 CH4 – Each molecular orbital is combination of ¼ s and ¾ p – Tetrahedral geometry, 109.5°, strong directional bonds sp3 Hybrid Orbitals Hybrid Orbitals • – – – – – • H 3 bonds, ⅓ s and ⅔ p H 1 p bond sp2 approximately 120° p perpendicular to axis out of the axis, more reactive Hybridization sp2 C2H4 sp2 Hybridization sp C2H2 – 2 hybrids ½ s + ½ p, – 2 pure p, H C C C H H sp2 CH sp Hybrid Orbitals Hybrid Orbitals • • H Benzene (sp2) C6H6 Valence bonding theory VB C – Each C uses 3 sp2 orbitals to form bonds with H and next C – Planar symmetrical hexagon, 120° – 6 e– in 6 p orbitals perpendicular to bonds form 3 bonds, 2 e– in each – 3 single and 3 double bonds • sp2 H C H C C H C H C 1s H Shortcomings p – Double bonds are not so stable – C–C 1.54 Ǻ; C=C 1.35 Ǻ – No isomeric compounds found • Resonance model – Resonance hybrid between structures (A) and (B) – 1.5 bonds between C atoms H H H H H H H (A) resonance H H H H (B) H Hybrid Orbitals • • Benzene (sp2) C6H6 Molecular orbital theory MO – system of delocalized e– – C bonds 1.40 Ǻ – Stability: “delocalization energy” – VSEPR • Valence Shell Electron Pair Repulsion Theory • Predicts the shapes of the molecules VB hybridizes the atomic orbitals first then overlaps the resulting hybrid orbitals by using LCAO. MO overlaps the atomic orbitals first by using LCAO followed by VSEPR concepts.