* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Angiotensin Converting Enzyme Inhibitors and
NK1 receptor antagonist wikipedia , lookup
Psychopharmacology wikipedia , lookup
Discovery and development of angiotensin receptor blockers wikipedia , lookup
Discovery and development of neuraminidase inhibitors wikipedia , lookup
Metalloprotease inhibitor wikipedia , lookup
Neuropharmacology wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Discovery and development of integrase inhibitors wikipedia , lookup
Discovery and development of ACE inhibitors wikipedia , lookup
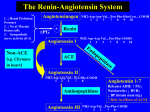
Current Pharmaceutical Design, 2003, 9, ???-??? 1 Angiotensin Converting Enzyme Inhibitors and Angiotensin II Receptor Antagonists in Experimental Myocarditis Lisa M. Godsel, Juan S. Leon and David M. Engman* Departments of Pathology and Microbiology-Immunology, Northwestern University Feinberg School of Medicine, Chicago, IL 60611, USA Abstract: Myocarditis is a disease whose pathogenesis is not completely understood and whose prevalence is likely underestimated. Individuals afflicted with this condition may be treated with agents that relieve symptoms arising from inflammation and concurrent cellular damage. One class of drugs commonly used in the treatment of myocarditis includes the angiotensin converting enzyme inhibitors, such as captopril, enalapril and lisinopril, and the angiotensin ΙΙ receptor antagonists, such as L-158,809 and losartan. The effects of these drugs on cardiomyopathy have been studied using a variety of animal models of heart failure and hypertension. However, less research has been done in the area of animal models of frank myocarditis. Here we review the use of angiotensin converting enzyme inhibitors and angiotensin ΙΙ receptor antagonists in animal models of myocarditis. We extend the implications of that published work by correlation with results from studies of other disease models and in vitro experiments that highlight the immunomodulatory potential of these compounds. The literature strongly suggests that aggressive therapy employing angiotensin converting enzyme inhibition and/or blockade of angiotensin ΙΙ receptors is beneficial. Treatment is useful not only for reducing complications associated with myocarditis, but also for downregulating the potential autoimmune component of disease—without increasing the levels of the infectious agent that may initiate the myocarditis. Key words: angiotensin converting enzyme, angiotensin II, renin-angiotensin system, immune modulation, myocarditis, autoimmunity. Trypanosoma cruzi infection is a major cause of myocarditis in Central and South America [9]. MYOCARDITIS Myocarditis, or inflammation of the heart, is formally defined as mononuclear cell infiltration of cardiac tissue, with or without myocyte necrosis, degeneration or fibrosis [1]. There are many potential etiologies of myocarditis and the prevalence of this group of diseases is probably underestimated. For example, in a study of 176 individuals with clinically suspected dilated cardiomyopathy, 14 were given a clinical diagnosis of borderline myocarditis, while 67 were found to have cardiac mononuclear cell infiltration upon biopsy [2,3]. A number of different cell types may comprise the tissue infiltrate, including CD4+ and CD8+ T lymphocytes, B lymphocytes, natural killer cells, neutrophils and macrophages [4-6] and there are distinct types of myocarditis in which one cell type is particularly abundant, including those predominated by lymphocytes, eosinophils, and giant cells [1]. It is estimated that 2,500 individuals develop myocarditis each year in the United States, the majority being children [7], and myocarditis is a major cause of sudden death in young persons [5]. There are a number of agents that may cause myocarditis, including bacteria, viruses and parasites, drugs and toxins among many others [1,3,5,8]. Viral infection is the most common cause of myocarditis in North America and Europe while Treatments for myocarditis mainly involve management of the associated disease complications, such as congestive heart failure, cardiogenic shock, conduction abnormalities, dysrhythmias and thromboembolism [10]. These typically involve the administration of diuretics, digitalis, beta blockers and vasodilators such as nitroglycerin, inotropic drugs and angiotensin converting enzyme (ACE) inhibitors [11]. In cases of diagnosed myocarditis, immunosuppressive therapy may be warranted. However, the effectiveness of this course of treatment is unpredictable and may be contraindicated, for example, in the setting of infection [5,12-20]. There is a wealth of literature comparing the relative efficacy of ACE inhibition and angiotensin ΙΙ receptor (AIIR) antagonism for the treatment of heart failure and hypertension [21]. For this reason, we will restrict our discussion to the comparatively small number of studies involving animal models of myocarditis. Interestingly, these reports suggest that ACE inhibitors and AIIR antagonists may be helpful in treating disease even in the presence of acute viral infection and, despite being potentially immunosuppressive, these agents do not increase the viral load. ACE AND ACE INHIBITORS *Address correspondence to this author at the Department of Pathology, Ward 6-175, 303 E. Chicago Ave., Chicago, IL 60611; Tel: 312-503-1288; Fax: 312-503-1265; E-mail: [email protected] 1381-6128/03 $35.00+.00 ACE is a bivalent dipeptidyl carboxyl metallopeptidase present in soluble form in bodily fluids and as a membrane © 2003 Bentham Science Publishers Ltd. 2 Current Pharmaceutical Design, 2003, Vol. 9, No. 3 bound form in endothelial, epithelial or neuroepithelial cells of several organs—the heart being the focus of this discussion. ACE is a key component of the reninangiotensin system in which angiotensinogen, synthesized by the liver and released into the blood, is cleaved by renin to generate the decapeptide angiotensin Ι (AI). ACE binds to AΙ and cleaves its C-terminal dipeptide, His-Leu, creating angiotensin ΙΙ (AΙΙ). ACE also cleaves the C-terminal dipeptide Phe-Arg from bradykinin, thus regulating the balance between the renin-angiotensin and kallikrein-kinin systems [22]. Generation of the octapeptide AΙΙ initiates a number of physiologic events associated with the reninangiotensin system, such as vasoconstriction. Although the renin-angiotensin system is endocrine, a number of tissues, including the heart, actually contain and synthesize components of the system. An outstanding review by Dostal and Baker discusses the evidence suggesting the presence of a functional cardiac-specific renin-angiotensin system [23]. Recently, a second ACE (ACE2) was identified in the vascular endothelial cells of the kidney and heart [24, 25]. ACE2 is hypothesized to regulate blood pressure but is more likely involved in heart function. Interestingly, ACE2 is insensitive to classical ACE inhibitors, including captopril. ACE inhibitors are drugs that inhibit the formation of AΙΙ from AΙ, as well as the breakdown of bradykinin, by binding to ACE via its peptide-binding pocket. A large number of ACE inhibitors is commonly prescribed today, including but not limited to captopril, enalaprilat, enalapril, lisinopril, quinapril, ramipril, trandolapril and zofenopril. AΙΙ is a potent vasoconstrictor, but may also be an important immunumodulator in its own right. Some of the immunomodulatory properties of AΙΙ are also briefly discussed below in the context of ACE inhibition. ACE inhibitors are commonly prescribed both as a first course of and as continued treatment for myocarditis, including autoimmune and infectious forms of the disease. While many experimental animal studies have explored the usefulness of modulating the renin-angiotensin system in heart failure and hypertension [20], little has been done to look at the effectiveness of the treatments in animal models of myocarditis. The few studies published suggest that ACE inhibitors are immunomodulatory drugs and their use in myocarditis may aid in reducing disease morbidity, even in the presence of viral or protozoan agents. These studies are detailed in the sections below, together with a summary of the research done to characterize the immunomodulatory mechanisms of these commonly prescribed vasodilators. AIIR AND THEIR ANTAGONISTS There are two subtypes of AΙΙR in humans, AT1R and AT 2 R [26] and, in rats, AT1 R has two subtypes, AT1A R and AT1BR [27]. AIIR are seven-transmembrane G proteincoupled receptors which mediate the physiologic actions of AΙΙ, such as vasoconstriction, hypertophy, proliferation of cardiac fibroblasts and production of extracellular matrix components [28]. Both AT1R and AT2R are expressed in the heart; however, AT1 R is the receptor through which AΙΙ exerts most of its effects by activating phospholipase C or inactivating adenylate cyclase [29]. AΙΙR have been reported to be increased in myocardial infarction [30], and models of Engman et al. cardiomyopathy [31-34], and hypertrophy [35-38]. Receptor antagonists, such as losartan and PD123319, interact with the transmembrane region of the receptor and inhibit the binding of AΙΙ to AT1R or AT2R, respectively. EXPERIMENTAL INFECTIOUS IMMUNE MYOCARDITIS MODELS AND AUTO- The most extensively studied models of myocarditis are viral models utilizing the cardiotropic encephalomyocarditis virus (EMCV) or coxsackievirus B3 (CB3). The diseases have three phases—acute, subacute, and chronic. The acute phase occurs within the first few days, during which death due to viral infection can occur without the presence of frank cardiac inflammation. The subacute phase occurs 4-14 days post infection and, during this time, heart failure is concurrent with inflammation and cardiac fibrosis. Inflammatory cells observed in cardiac lesions first include natural killer cells. By 7 days post infection macrophages and lymphocytes are observed. The presence of the lymphocytes correlates with the most severe cardiac damage. In the chronic stage of the disease, virus cannot be cultured from tissue; however, persistent cardiac damage is observed. A variety of immunosuppressive agents have shown no favorable effects and, in fact, led to increased damage resulting from an increased viral titer [5,39]. Experimental autoimmune myocarditis (EAM) is induced in susceptible strains of mice upon immunization with the cardiac myosin α heavy chain [40]. This disease model is useful in that anti-cardiac autoimmune responses can be studied separately from those directed against an infectious agent. Interestingly, immune responses against cardiac antigens, cardiac myosin in particular, have been observed in human inflammatory heart disease [41-47], making myosin a relevant and effective antigen for disease induction in the mouse model. Recent studies utilizing EAM have shown that myosin-specific immunosuppression is possible and an avenue worth pursuing [48,49]. EAM is histologically similar to human myocarditis, with myocyte swelling and necrosis accompanied by mononuclear cell infiltration and fibrosis. Studies in animals have shown that EAM is a T cell mediated disease, requiring both CD4+ and CD8+ subsets [50-54]. B cells are not vital for antigen presentation in EAM and autoantibodies are not necessary for the progression of myocarditis [55]. Another model of infectious myocarditis is that induced in mice upon infection with the protozoan Trypanosoma cruzi. T. cruzi is the agent of human Chagas heart disease, a chronic, progressive, and fibrosing myocarditis of variable degree. Upon infection and entry into the bloodstream, T. cruzi invades cardiac myocytes, replicates in the cytoplasm, and is released into the interstitium upon myocyte lysis. The acute phase of Chagas heart disease is characterized by a focal inflammation in the myocardium composed of mononuclear cells (lymphocytes, macrophages, and plasma cells), mast cells, and granulocytes. Intense tissue parasitosis is typically found in both cardiac and skeletal muscle. The acute myocarditis resolves after several weeks. Entry into the chronic phase is characterized by development of a globose heart with a rounded apex due to biventricular hypertrophy ACE and Myocarditis Current Pharmaceutical Design, 2003, Vol. 9, No. 3 and chamber dilatation. The exudate in chronic chagasic myocarditis is mainly composed of lymphocytes and, to a lesser degree, by macrophages, eosinophils, plasma cells, neutrophils, and mast cells. T lymphocytes predominate, with CD8+ T cells outnumbering CD4+ cells 3 to 1 (reviewed in [56]). In contrast to what is found in the acute phase, parasites are often undetectable in the hearts during the chronic phase. There is a variable degree of myofibrillar destruction and replacement by connective tissue, leading to progressive fibrosis. from our laboratory and others [57] have addressed the effects of captopril in a non-infectious, purely autoimmune murine model of the disease. The earliest studies focused on the efficacy of captopril in treating an animal model of viral myocarditis. Rezkalla et al. published work on the effect of captopril on both early (6 days of treatment) and late (20 or 30 days of treatment) models of CB3 myocarditis [58]. In the early treatment protocol, mice received captopril from day 1 through day 6, while in the late model they did not receive captopril until 10 days post infection. Mice treated in both the early and late protocols had significant reduction in heart weight and heart weight/body weight ratio compared to control animals. Viral titers were the same in the captopril treated and control animals. This indicates that any immunosuppressive effect of captopril did not affect the antiviral immune response. Hearts from animals given the early protocol had less necrosis and calcification and decreased inflammation; however, hearts from the late protocol with treatment at either 20 or 30 days post infection ACE INHIBITORS AND AIIR ANTAGONISTS IN EXPERIMENTAL MYOCARDITIS Captopril, enalapril and temocapril are the only ACE inhibitors that have been studied in animal models of myocarditis. The earliest work focused on CB3 and EMCV myocarditides and is summarized in Table 1. Recent studies Table 1. Agent CB3 EMCV Use of ACE Inhibitors and AIIR Antagonists in Myocarditis Models 'Viral Titer or Parasitemia Heart Wt or Heart Wt:Body Wt Ratio LV Wall Thickness and Cavity Diameter Myofibrillar Diameter Inflammation Necrosis Calcification Fibrosis Ref Captopril treatment 1-6 days post inoculation nc ↓ nd nd ↓ ↓ ↓ nd [58] Captopril treatment starting 10 days post inoculation nc ↓ nd nd nc nc nc nd [58] Captopril treatment 3 days post inoculation nd ↓ nd nd nc ↓ ↓ nd [59] Captopril treatment 10-30 days post inoculation nd ↓ nc ↓ ↓ ↓ nc ↓ [64] Captopril treatment 30-60 days post inoculation nd nc nc nc nc nc nc nc [64] Captopril treatment from day of inoculation nd ↓ nd nd ↓ ↓ ↓ nd [60] TCV116 (3 mg/kg dosage) nc ↓ nd nd ↓ ↓ ↓ nd [66] Captopril nd ↓ ↓ nd ↓ ↓ nd nd [62] Enalapril nd nc nc nd nc ↓ nd nd [62] Inoculation and Treatment Protocols 3 Losartan nd ↓ ↓ nd nc nc nd nd [62] Captopril treatment from 4-16 weeks post inoculation nd ↓ ↓ ↓ nd nd nd ↓ [63] Losartan treatment from 4-16 weeks post inoculation nd ↓ ↓ ↓ nd nd nd nc [63] Captopril treatment 7-21 days post inoculation nd ↓ nd ↓ ↓ ↓ nd nd [65] Enalapril treatment 7-21 days post inoculation nd ↓ nd ↓ nc nc nd nd [65] L-158,809 treatment 7-21 days post inoculation nd ↓ nd ↓ ↓ ↓ nd nd [65] Myosin in CFA Temocapril treatment from day of immunization na ↓ nd nd ↓ nd nd nd [57] Myosin in CFA Temocapril treatment 15-21 days post immunization Na nc nd nd nc nc nd nd [57] Myosin in CFA Captopril treatment from day of immunization na ↓ nd nd ↓ ↓ nd ↓ this review T. cruzi Captopril treatment from day of inoculation nc nc nd nd ↓ ↓ nd ↓ this review na, not applicable; nc, no change from controls; nd, not determined; ↓ reduction compared to controls. 4 Current Pharmaceutical Design, 2003, Vol. 9, No. 3 were not statistically different in any of these three criteria. The effects of captopril on virus-specific immunity were not studied. The same authors published on another short-term model of CB3 myocarditis (9 days of infection) [59]. In that model, captopril treatment was initiated 3 days after infection and continued until the completion of the experiment. The heart weights of the captopril treated animals were different from those of the non-treated controls; however, this difference was not addressed carefully by determining the heart weight/body weight ratio. Myocyte necrosis and calcification were also decreased upon captopril treatment, although the degree of inflammation was unchanged. These studies suggest that captopril treatment can be beneficial in decreasing cardiac damage. Based on the finding that captopril may decrease inflammation, it would be useful to know whether the viral titer increased in the captopril treated animals. Taken together, the results of these papers seem to indicate that there is a narrow window in which captopril treatment should be initiated early to have the most beneficial effect on the inflammatory process, although later treatment initiation is still of some utility. Captopril and some other ACE inhibitors contain sulfhydryl groups which have been hypothesized to be involved in the drug’s effectiveness [60,61]. The EMCVinduced myocarditis model was used to compare the effects of captopril and N ,2-mercapto-propionyl glycine, a sulfhydryl-containing amino acid derivative without ACE inhibiting properties [60]. In this paper the authors reported that both drug treatments significantly increased survival and decreased the amount of cellular infiltration, myocyte necrosis and calcification in the hearts of the animals. Body weights of the treated animals were significantly higher than those of controls, as were the heart weight/body weight ratios. The authors made the comparison between these two drugs in an attempt to explore the effectiveness of the sulfhydryl group in mediating captopril’s protective effects beyond ACE inhibition. In autoimmune myocarditis, administration of temocapril to myosin-immunized mice at day 0, but not 21, post immunization, decreased heart weight/body weight ratios, pericardial effusion, and macroscopic and microscopic histologic scores [57]. The authors suggested that this effect may be due to thioredoxin, a protein involved in protecting cells from oxidative stress. This result may support the findings of other groups that suggest that the sulfhydryl group of ACE inhibitors ameliorate heart function through a redox mechanism. Araki et al. then studied the effects of captopril and enalapril in treating EMCV-induced myocarditis and compared their effects to those of losartan, an AΙΙR antagonist [62]. Heart weights and heart weight/body weight ratios were reduced and left ventricular wall thickness and cavity dimension were both decreased by captopril and losartan treatment, but not by enalapril. Captopril reduced cardiac inflammation and myocyte necrosis, while enalapril only decreased myocyte necrosis. Losartan did not have any effect on these two parameters, even though several doses were tested. Therefore, these studies suggest that, while the effect of captopril and enalapril on the renin-angiotensin system is the same, there are some differences in the Engman et al. immune system regulation. These changes in the immune system resulting from captopril treatment, at least in the EMCV model, are not simply due to the lack of circulating AΙΙ in the animals. In another study, losartan and captopril treatment were compared in a long term model of EMCV myocarditis [63]. Mice received the drugs from 4 weeks after viral inoculation to the termination of the experiment at 16 weeks post infection. Losartan and captopril had similar effects on all parameters tested—heart weight, left ventricular thickness, left ventricular cavity dimension and myocardial fiber diameter. The two drugs differed in their effect on fibrosis. Only captopril lowered the degree of fibrosis, an activity of this drug that has been explored by others in myocarditis and other disease models. Takada et al. used CB3 induced myocarditis as a model to study the effect of captopril on fibrosis [64]. The authors looked at both the inflammatory phase, with captopril being administered on days 10 through 30 post infection, or the fibrotic phase, with the drug administered on days 30 through 60 post infection. In the inflammatory phase, survival was higher in the captopril treated group and inflammation, necrosis and fibrin deposition were all decreased. Furthermore, connective tissue architecture was maintained, myocyte hypertrophy was decreased and the shift of myosin isoforms from α to β was prevented. In this case, calcification was not statistically different from that of controls. The fact that inflammation is decreased contrasts with results of Rezkalla et al., who observed decreased inflammation and calcification only when captopril was administered at the time of infection, and not when the drug was administered 10 days post infection [58]. The disparity may be explained by differences in the specific experimental protocols, the mouse and/or virus strain, the number of plaque-forming units of virus administered or the dosage of drug. In the fibrotic phase of disease the only significant difference in the results of Rezkalla et al. was that interstitial reticulin fibers showed decreased thickening in treated animals compared to controls. This indicated that captopril afforded protection from virally mediated damage, even if treatment was begun 10 days after the infection was initiated. Baba et al. published a study which addressed the effects of the ACE inhibitors captopril and enalapril and the AΙΙR antagonist L-158,809 on cardiac damage after EMCV infection [65]. Mice were given the drugs for 14 days starting 7 days post inoculation of virus. Heart weight/body weight ratios and myofibrillar hypertrophy were significantly decreased in the animals treated with any of the three agents compared to untreated controls. Myocardial necrosis and inflammation were reduced in the captopril and the L158,809 treated animals, but not in the enalapril treated mice. The AΙΙR antagonist, but not either ACE inhibitor, also reduced the amount of another potent vasoconstrictor, endothelin-1. These findings illustrate that ACE inhibition and AΙΙR blockade are not entirely interchangeable approaches and that treatment regimens should be individualized. There are only four reports in the literature describing AΙΙR blockade in myocarditis and three involving L-158,809 and losartan, which were mentioned above. Mice were ACE and Myocarditis treated with a variety of concentrations of the AIIR blocker TCV-116 one day before or two days (1 or 10 mg/kg and 0.3 or 3 mg/kg, respectively) after EMCV inoculation [66]. 3 mg/kg treatment resulted in a significantly lower heart weight, myocyte necrosis, calcification and cellular infiltration than in untreated control animals. However, the drug at this concentration did not have any effect on viral 1sp titer. While the findings in this particular paper are intriguing, it is difficult to compare the groups because of the very different amount of the drug used. These data correlate well with those in the EMCV model addressing the effects of L-158,809 [65], but do differ from those for losartan, in which there was no effect on inflammation or necrosis [62]. As mentioned above for the CB3 papers, the different results obtained in studies of AIIR blockade could be due to variations in the experimental methods and reagents used in each study. It is noteworthy that, in the above experiments, treatment with ACE inhibitors or AΙΙR antagonists did not lead to an increase in viral load, as might be expected if the agents are immunosuppressive. However, it is possible that the agents have antiviral activity. The direct effects of the treatments on the virus have not been examined in any detail. One in vitro study examined the effects of captopril or losartan treatment on herpes simplex virus type 2 infection of cardiac neonatal myocytes. Losartan reduced cellular damage, as measured by lactate dehydrogenase release into the medium, and viral Current Pharmaceutical Design, 2003, Vol. 9, No. 3 5 release. However, the rate of viral replication was not affected. Captopril had no effect on the same parameters, so while this drug may not be as efficacious as losartan, it does not increase the damage or viral load as compared to controls [67]. Work from our laboratory strongly suggests that captopril is useful for the prevention of autoimmune myocarditis, as well as for myocarditis resulting from infection with the cardiopathogenic protozoan parasite Trypanosoma cruzi. A/J and BALB/cJ strains of mice immunized with syngeneic cardiac myosin develop a severe, diffuse inflammatory disease specifically localized to the cardiac tissue and absent from skeletal muscle and smooth muscle. Captopril prevented development of increased heart weight, heart weight/body weight ratio, cardiac inflammation and cardiac fibrosis (Fig. (1)). The captopril treated animals also failed to develop myosin-specific cellular immunity in response to myosin-immunization as measured by delayed-type hypersensitivity. Further experiments indicate that this immunosuppression was not cardiac specific, since ovalbumin-specific cellular immune responses were also decreased in ovalbumin-immunized animals treated with captopril (data not shown). Therefore, it appears that both ACE inhibitors and AIIR antagonists may be beneficial when myocarditis is suspected, since they seem to suppress the complications Fig. (1). Captopril reduces myocardial inflammation and fibrosis in A/J mice immunized with cardiac myosin or phosphate-buffered saline (PBS) in complete Freund’s adjuvant. Representative cardiac sections of captopril treated (75 mg/L in the drinking water beginning at first immunization) and untreated mice at 28 days post treatment. Sections are at 40X and are stained with Masson’s trichrome or hemotoxylin and eosin. 6 Current Pharmaceutical Design, 2003, Vol. 9, No. 3 Engman et al. associated with myocarditis without increasing disease severity. They have the added advantage of decreasing cardiac-specific inflammation without promoting increased replication of the infectious agent. From the work cited in this section, it is clear that critical information on the use of these agents in myocarditis is lacking and that there are many aspects of both ACE inhibition and AIIR blockade that should be investigated more extensively. The remainder of this review will focus on studies of the immunomodulatory effects of both ACE inhibitors and AIIR antagonists on immune cell function and correlate those findings with the results described above. ACE INHIBITORS IN CHAGAS HEART DISEASE ACE inhibitors are also used to treat another prevelant cardiomyopathy, Chagas heart disease (CHD), caused by infection with the parasite Trypanosoma cruzi. CHD is a common cause of cardiac death in Latin America and may manifest as congestive heart failure, cardiac dysrhythmia or thromboembolism. Once heart failure develops in CHD patients, life expectancy is reduced to a few years and sudden death occurs in approximately 40% of patients [68]. Management of clinical CHD is based on the treatment of other cardiomyopathies. CHD patients with congestive heart failure respond to routine management, including sodium restriction and treatment with diuretics, digitalis, and ACE inhibitors. Despite the routine administration of ACE inhibitors to patients with CHD, only a few pilot studies have been conducted to test their potential benefits. These studies are summarized in Table 2. Captopril and enalapril, two ACE inhibitors, seem to improve cardiac function and are well tolerated by Chagas patients; at least 90% of patients do not develop side effects [69,70]. Patients with cardiomyopathy, including CHD patients, were given captopril (75 mg/day) for 12 weeks and these individuals exhibited a significant decrease in heart rate and objective improvement of cardiac function in 98 (85.2%) patients [69]. However, no conclusions can be drawn about the CHD patients since the results of their treatment were not presented separately. ACE inhibitors may improve cardiac function in CHD patients by interfering with the renin-angiotensin system. While ACE is probably the main target of these inhibitors, Table 2. ACE activity levels were not measured in any of these studies. ACE inhibitors can also affect renin levels, though they are believed to operate downstream of the renin biosynthetic pathway. Renin levels have been observed to be higher in CHD patients with heart failure than in those with asymptomatic CHD [71]. One study of CHD patients reported an increase in plasma renin levels upon administration of captopril for 6 weeks [70], while a second study reported decreased renin levels upon administration of enalapril over 96 hours [72]. The change in renin levels induced by ACE inhibitors probably influences AΙΙ levels and cardiac function. Interestingly, one study reported that the ACE inhibitor captopril enhanced T. cruzi invasion of host cells in vitro and therefore may affect cardiac function in the host [73]. Captopril blocks the degradation of bradykinin, which was shown to increase infection of host cells by T. cruzi. However, there are no published reports of ACE inhibitors affecting the parasite burden of CHD patients. Lastly, these ACE inhibitors may affect cardiac function through the parasympathetic and sympathetic systems, since the levels of neurohormones such as norepinephrine were affected. In conclusion, preliminary reports suggest that cardiac function in individuals with CHD may be improved by ACE inhibitor therapy. The mechanisms by which ACE inhibitors may provide this benefit are unknown but may include modulation of the renin-angiotensin system and the parasympathetic/sympathetic systems. Large scale controlled studies on morbidity and long term survival of CHD patients treated with ACE inhibitors are necessary to confirm the benefits of ACE inhibitors in CHD and to identify specific populations best suited to receive such therapy. CAPTOPRIL IN EXPERIMENTAL CHAGAS HEART DISEASE Preliminary evidence in our laboratory suggests that captopril treatment also reduced myocarditis in an experimental model of acute Chagas heart disease. Captopril treatment of A/J mice infected with T. cruzi resulted in a decrease in cardiac inflammation and cardiac fibrosis (Fig. (2)). However, the reduction in inflammation and fibrosis was not associated with a decrease in heart weight or heart weight/body weight ratio compared to infected controls that did not receive captopril. Interestingly, infected mice treated Use of ACE Inhibitors and AIIR Antagonists in Clinical Chagas Disease Ace Inhibitor Type Size Dose mg/day Length Effects/Comments Ref Captopril Single blind, cross-over 18 150 12 weeks (6 weeks treatment) ↓Heart rate, ↓Ventricular couplets (ns), ↓Nonventricular tachycardia (ns),↑Plasma renin, ↓Urinary, norepinephrine, Systolic/Arterial blood pressure (nc) [70] Enalapril/ Captopril Prospective Cohort 56 “conventional doses” 2 years No association with mortality (ns) [139] Enalapril Intervention 13 5 96 hours ↓Functional Class, ↓Weight, ↓Plasma Norepinephrine, ↓Aldosterone, ↓Renin, Systolic/Arterial blood pressure (ns) [72] Enalapril Intervention 20 5-10 8 weeks ↓ E/A relationship (improved diastolic function) [140] nc, no change from controls; ns, not significant; ↓ reduction compared to controls. ACE and Myocarditis Current Pharmaceutical Design, 2003, Vol. 9, No. 3 7 Fig. (2). Captopril reduces myocardial inflammation and fibrosis in A/J mice acutely infected with T. cruzi. Representative cardiac sections of captopril treated (75 mg/L in the drinking water beginning at infection) and untreated mice at 28 days post treatment. Sections are at 40X and are stained with Masson’s trichrome or hemotoxylin and eosin. Arrowheads indicate T. cruzi pseudocysts. with increasing amounts of captopril had higher mortality. In addition, captopril treated mice exhibited reduced anti-T. cruzi cellular immune responses as measured by delayed type hypersensitivity, similar to the immunosuppresive effect observed in captopril-treated, myosin-immunized mice. These results suggest that, while captopril treatment reduces inflammation in T. cruzi induced myocarditis, captopril may also enhance T. cruzi - induced mortality. ACE INHIBITORS IN OTHER AUTOIMMUNE AND INFECTIOUS DISEASE MODELS A number of other disease models, both infectious and autoimmune, have yielded more information regarding the effects of ACE inhibitors on the immune response. In an animal model of multiple sclerosis, experimental autoimmune encephalomyelitis, captopril treatment resulted in decreased clinical disease severity and a suppressed and shortened duration of the disease. In vitro studies showed that lymphocytes from treated animals had a diminished response to myelin basic protein and to the mitogen concanavalin A (Con A) [74]. Several papers describe the use of captopril in a number of granulomatous inflammation models. The drug reduced the size of liver granulomas in murine schistosomiasis [75], but not the size of hypersensitivity granulomas induced by bovine serum albumin-coated sepharose beads. Since the latter granulomas contained no ACE, the paper suggested that the immunomodulatory effects of captopril may be due, at least in part, to the inhibition of ACE. In another study, captopril was used to decrease lung inflammation and splenomegaly in Bacille Calmette Guerin-induced granuloma [76]. In vitro the drug did not affect the macrophage migration nor did it change the chemotactic activity of isolated granulomas. In yet another paper the granulomatous response to Histoplasma capsulatum was modulated by administration of captopril with different findings from any of those reported above. With ACE inhibition present during the early stages of the infection, the clinical severity of infection and the histopathologic changes in mice were significantly worsened and the growth of the yeast in livers and spleens was increased [77]. If captopril was first administered later during the resolution phase of the infection, there was no effect on the healing of the granulomas, but the drug did not cause a relapse of infection. Some in vitro experiments demonstrated that captopril treatment of normal animals did not result in suppression of splenocyte responses to the mitogens Con A or phytohemagglutinin in vitro or delayed type hypersensitivity. The somewhat contradictory results of 8 Current Pharmaceutical Design, 2003, Vol. 9, No. 3 Engman et al. these in vitro experiments may be due to differences in the specific ACE inhibitor employed, or to differences in the dosage and treatment regimen used both in the animals and in the culture dish. The presence of AΙΙ during the course of infection may therefore affect the outcome, depending on the type of infection involved, and a decrease in AΙΙ may be the method through which ACE inhibition decreases the size of the granulomas. The protective effects of AΙΙ against infection are presented in a paper studying bacterial peritonitis in which treatment of rats treated with AII inhibitors resulted in a decreased abscess size and an increase in host resistance [78]. These data were correlated with in vitro experiments showing that AΙΙ treatment increased respiratory burst of rat peritoneal macrophages and increased phagocytosis of rat macrophages and human peripheral blood mononuclear cells. Thus, the presence of AΙΙ can increase the activation of the immune response against an infectious agent, while the inhibition of AΙΙ production via ACE inhibition or AΙΙR antagonism may decrease the activation of immune cells. Information is available regarding the effects of captopril and other ACE inhibitors and AΙΙR antagonists that differ enough from each other to suggest that not all of the effects mediated by ACE inhibitors can be explained simply by a lack of AΙΙ in the circulation or resident in tissues. The section below strives to summarize the research on a variety of indicators of normal inflammatory cell function. IMMUNOMODULATORY EFFECTS OF INHIBITORS AND AIIR ANTAGONISTS ACE There are a number of ways that ACE inhibitors and AΙΙR antagonists may modulate the immune response. Several of the processes that may be affected include chemotaxis, motility and adhesion, differentiation, activation and cytokine/chemokine production. There is also a large base of research demonstrating an involvement of AΙΙ in basic immunity, suggesting potential utility for ACE inhibitors and AΙ Ι R antagonists in modulating inflammation. Much of the research has focused on cellular adhesion and chemotaxis and how ACE inhibitors and AΙΙR antagonists modulate these processes. For example, both types of agent may inhibit mononuclear cell infiltration, either because of drug-induced decrease in expression of adhesion molecules on the mononuclear, endothelial or interstitial cells or because of decreased chemokine production. A variety of in vivo and in vitro studies have demonstrated that AII is involved in the chemotaxis and adhesion of monocytes and macrophages. Angiotensin knockout animals have decreased production of MCP-1 [79] AIII is an AII processing product that also increased MCP-1 [80]. ACE inhibition resulted in decreased MCP-1 levels in a model of atherosclerosis [81,82] as well as in patients suffering myocardial infarction, which correlated with a decreased accumulation of macrophages in heart tissue [83]. In addition, AΙΙ has been shown to induce monocyte migration [84], leukocyte rolling, adhesion and extravasation while AΙΙR antagonists inhibit these processes [85]. AΙΙ induced leukocyte-endothelial cell interactions in vivo [86]. AΙΙR antagonists and ACE inhibitors reduced the amount of neutrophil [87,88] and macrophage [89] recruitment and decreased neutrophil [90], polymorphonuclear cell [91,92] and leukocyte [93] adhesion. Enalapril decreased ICAM-1, VCAM-1, MCP-1 levels in circulating adhesion molecules in serum of patients [94]. In some of the studies, differences among the results of AΙΙR antagonism and ACE inhibition were highlighted. For example, study of the effects of captopril on neutrophils showed that treatment did not affect neutrophil adherence [90] and, in fact, actually might enhance neutrophil migration [95] and activation [96], in contrast to the results detailed above. In another study, treatment decreased the activated circulating and endothelium-adherent monocytes [97]. AIIR antagonists prevented increased expression of ICAM-1 and VCAM-1, which correlated with a decrease in macrophage infiltration [98,99]. Other studies did not support this conclusion, however, possibly because a different agent was employed [100,101]. A ΙΙ therefore may upregulate chemotactic factors important for attracting immune cells to the affected tissue, and may induced expression of molecules that enable the cells to adhere to endothelium. Administration of ACE inhibitors or AΙΙR antagonists decreases the amount of circulating and tissue AΙΙ and may decrease inflammation in this manner. Another way that ACE inhibitors and AΙΙR antagonists may modulate the immune response is via their effects on immune cell proliferation and differentiation. AΙΙ has been shown to act through AT1 R to stimulate the proliferation of splenic lymphocytes [102,103]. Interestingly, ACE activity, AΙΙ release, and AT1 R and AT 2R expression all increased when the human leukemia cell line THP-1 is induced to differentiate into a macrophage [104]. The majority of the ACE inhibition studies that focus on cell proliferation and differentiation utilized captopril, while a small number utilized enalapril and enalaprilat. Unfortunately, many of the results are contradictory. Captopril inhibited the proliferation of hematopoietic stem cells and progenitors [105] and also reduced proliferation of the stem cells in vivo [106]. In contrast, the drug stimulated the proliferation of both human [107] and rat [108] lymphocytes in vitro, but proliferation of lymphocytes derived from captopril treated rats was inhibited. In a mouse model of lupus, captopril was used to inhibit T cell stimulation, but enalapril did not have this effect [109]. However, in another study, captopril increased Con Astimulated T cell proliferation while enalapril and enalaprilat inhibited the proliferation [110]. Finally, T cells were stimulated when cocultured with a low concentration of serum from patients treated with either captopril or enalapril, but were inhibited when the serum concentration was high [111]. Because AΙΙ stimulates lymphocyte proliferation, it follows that ACE inhibitors would decrease proliferation of immune cells through reduction of AΙΙ. There is a suggestion that all these effects are directed at least in part at macrophages and there may be some effects of the drugs that are independent of AΙΙ. Another way that ACE inhibitors and AT1R and AT2R antagonists might modulate the immune response is via ACE and Myocarditis alteration of cytokine release. AΙΙ induces transcriptional activation of a number of cytokine genes. ACE inhibitors and AT1 R and AT2 R antagonists might therefore inhibit cytokine secretion by decreasing the amount or antagonizing the effects of AΙΙ. AΙΙ increases the transcription of IL-6 in macrophages, which can be inhibited by AT1R antagonists [112,113]. This decrease in IL-6 production has also been observed in vivo in a model of chronic heart failure and myocardial infarction [114,115]. Quinapril treatment in a model of myocardial infarction reduced expression of IL-1β, IL-5, IL-6, and TNF-α. Captopril suppressed IL-1β induced production of IL1-α and TNF-α in peripheral blood [116]. Interestingly, captopril even increased production of IL-1Rα [113]. ACE inhibition was also shown to decrease the amount of IL-2, IL-12 and IFN-γ produced by T cells [117, 118]. In contrast, murine T cells secreted higher levels of IL2 upon captopril treatment after Con A stimulation [119]. Peripheral blood monocytes release TNF-α in response to AII treatment—an effect blocked by AT1R antagonism and ACE inhibition [113,116,120,121]. However, not every ACE inhibitor was able to block TNF-α expression by the same cell populations [116]. ACE inhibitors were also shown to be more efficient at decreasing the plasma concentration of TNF-α than are AΙΙR antagonists [122]. These results raise the point that the activities of ACE inhibitors are not all directly related to their effects on AΙΙ. Caution must be taken when analyzing the effects of these agents. In some cases, a large amount of drug is needed to obtain a decrease in cytokine production and the observed in vitro responses may therefore not be physiologically relevant [113,116,123]. TGF-β is a cytokine that is regulated by AΙΙ and is decreased by ACE inhibition and AIIR antagonism [124-127]. While cytokines are markers of cellular activation, there are other important markers of activation that have also been analyzed. For example, AΙΙ has been shown to increase macrophage phagocytosis [128] and the adherence of peripheral blood monocytes to endothelial cell monolayers [120]. AΙΙ increased cytosolic calcium in peripheral blood mononuclear cells, which could be blocked by the AT1R antagonist losartan [130]. Along these lines, treatment of hypertensive rats with the ACE inhibitor captopril significantly lowered the intracellular calcium in thymocytes, which correlated with a decrease in blood pressure [130]. The studies mentioned above support the hypothesis that ACE inhibition and AΙΙR antagonism are involved in the downregulation of the immune response. This downregulation may be mediated in part by the simple fact that AΙΙ is not produced, as in the case of ACE inhibition, or its activity is inhibited, as in the case with receptor antagonism. However, there are differences in the way that ACE inhibitors function compared to receptor antagonists and even how drugs within the same class effect immune cell function. Some papers even showed that the activities of some types of immune cells are actually enhanced by treatment with ACE inhibitors. ACE inhibitors have also been shown to increase the activation of neutrophils and mast cells as demonstrated by myeloperoxidase, lysozyme and histamine release [96,131,132]. These results indicate that the actions of these drugs are more complex than simply to reduce AΙΙ production and suggest that each drug needs to be analyzed individually. Certainly one must be aware that Current Pharmaceutical Design, 2003, Vol. 9, No. 3 9 an effect observed for one inhibitor or antagonist cannot be assumed to be the same for other agents. Underlying all of the data in the above reports is the possibility that ACE inhibitors and the receptor antagonists modify the immune response via cell signaling pathways. Certainly there are results suggesting that AΙΙ is involved in cell signaling and, in the most obvious scenario, ACE inhibitors and AT1R and AT2R antagonists could modulate the immune response by decreasing the concentration of AΙΙ available for signaling. AΙΙ-mediated degradation of IκB and activation of NFκB increased transcription and translation of VCAM-1 and ICAM-1, which are important for recruitment of leukocytes to inflamed tissues [1-3]. This upregulation may be due, at least in part, to the AΙΙ-induced production of endothelin-1 [133-135]. Angiotensin III, a product of AΙΙ cleavage, increases the production of MCP-1 via NFκB activation [80]. AΙΙ has also been shown to augment tyrosine kinase activity [136]. Interestingly, captopril treatment has been shown to inhibit pp60c-src tyrosine phosphorylation in human mesanglial cells in vitro [137]. Studies using ACE inhibitors and an AT1 R antagonist make the case for cell signaling even stronger as NFκB activation of macrophages and vascular smooth muscle cells is decreased upon treatment with these agents [81,82]. ACE inhibition with captopril, idapril, fosinopril, and losartan all reduced the expression of tissue factor in monocytes via the inhibition of NFκB translocation to the tissue factor promoter [138]. CONCLUSIONS ACE inhibitors and AΙΙR antagonists are extremely useful in the management of heart disease. However, little has been done to study the effects of these drugs in myocarditis specifically. There is a concern that use of these agents will decrease the immune response against an infectious agent that has induced the heart damage and/or that has precipitated the inflammation against self proteins (autoimmunity). There is cause for concern, since contradictory information regarding the effects of AΙΙ and its inhibitors on the immune response continues to be generated. However, the general theme arising from the papers on this topic suggests that these drugs may not increase the proliferation of an infectious agent and can decrease the morbidity of the inflammatory heart disease. The amount and type of ACE inhibitor or AΙΙR antagonist used may make a difference in the effects observed on the immune system. Certainly there are differences between the ACE inhibitors and the AΙΙ receptor antagonists and between drugs within either category, suggesting that each has its own specific effects on the immune system beyond inhibition of AΙΙ activity by decreasing the amount of AΙΙ or by blocking its effects. Some of the effects include chemotaxis and motility, the production of cytokines important in activation and proliferation, the accumulation of bradykinin and prostaglandin production and the deposition of extracellular matrix. With so many effects attributed to such widely administered medications, further research is necessary to fully understand the impact of these agents. 10 Current Pharmaceutical Design, 2003, Vol. 9, No. 3 ACKNOWLEDGEMENTS Research in our laboratory was supported by grants and fellowships from the National Institutes of Health and the American Heart Association. We thank Drs. William Ward, Agostino Molteni and Carl Waltenbaugh for helpful advice and assistance. Engman et al. [11] Dec, G. W.; DeSanctis, R. W. In Scientific American Medicine, D.C. Dale; D.D. Federman, eds.; Scientific American Publisher: New York, NY, 1990. [12] Aretz, H. T.; Billingham, M. E.; Edwards, W. D.; Factor, S. M.; Fallon, J. T.; Fenoglio, J. J., Jr.; Olsen, E. G.; Schoen, F. J. Am. J. Cardiovasc. Pathol., 1987, 1, 3-14. [13] McCarthy, R. E., 3rd; Boehmer, J. P.; Hruban, R. H.; Hutchins, G. M.; Kasper, E. K.; Hare, J. M.; Baughman, K. L. N. Engl. J. Med., 2000, 342, 690-5. [14] McNamara, D. M.; Rosenblum, W. D.; Janosko, K. M.; Trost, M. K.; Villaneuva, F. S.; Demetris, A. J.; Murali, S.; Feldman, A. M. Circulation, 1997, 95, 2476-8. [15] Bozkurt, B.; Villaneuva, F. S.; Holubkov, R.; Tokarczyk, T.; Alvarez, R. J., Jr.; MacGowan, G. A.; Murali, S.; Rosenblum, W. D.; Feldman, A. M.; McNamara, D. M. J. Am. Coll. Cardiol., 1999, 34, 177-80. [16] O'Connell, J. B.; Mason, J. W. West. J. Med., 1989, 150, 431-5. [17] Grogan, M.; Redfield, M. M.; Bailey, K. R.; Reeder, G. S.; Gersh, B. J.; Edwards, W. D.; Rodeheffer, R. J. J. Am. Coll. Cardiol., 1995, 26, 80-4. [18] Latham, R. D.; Mulrow, J. P.; Virmani, R.; Robinowitz, M.; Moody, J. M. Am. Heart J., 1989, 117, 876-82. [19] Parrillo, J. E.; Cunnion, R. E.; Epstein, S. E.; Parker, M. M.; Suffredini, A. F.; Brenner, M.; Schaer, G. L.; Palmeri, S. T.; Cannon, R. O., 3rd; Alling, D.; Wittes, J. T.; Ferrans, V. J.; Rene Rodriguez, E.; Fauci, A. S. N. Engl. J. Med., 1989, 321, 1061-8. [20] McNamara, D. M.; Holubkov, R.; Starling, R. C.; Dec, G. W.; Loh, E.; Torre-Amione, G.; Gass, A.; Janosko, K.; Tokarczyk, T.; Kessler, P.; Mann, D. L.; Feldman, A. M. Circulation, 2001, 103, 2254-9. ABBREVIATIONS ACE = Angiotensin converting enzyme AI = Angiotensin I AII = Angiotensin II AIII = Angiotensin III AIIR = Angiotensin II receptor AT1R = Angiotensin II receptor 1 AT2R = Angiotensin II receptor 2 CHD = Chagas heart disease CHF = Congestive heart failure CB3 = Coxsackievirus B3 Con A = Concanavalin A EAM = Experimental autoimmune myocarditis EMCV = Encephalomyocarditis virus REFERENCES [1] Pisani, B.; Taylor, D. O.; Mason, J. W. Am. J. Med., 1997, 102, 459-69. [21] Wollert, K. C.; Drexler, H. Cardiovasc. Res., 1999, 43, 838-49. [2] Kuhl, U.; Noutsias, M.; Schultheiss, H. P. Eur. Heart J., 1995, 16, 100-6. [22] Skidgel, R. A.; Erdos, E. In The Renin-Angiotensin System, J.I.S. Robertson; M.G. Nicholls, eds.; Raven Press, Ltd.: New York, NY, 1993, pp. 10.1-10.10. [3] Caforio, A. L.; McKenna, W. J. Drugs, 1996, 52, 515-25. [23] Dostal, D. E.; Baker, K. M. Circ. Res., 1999, 85, 643-50. [4] Kearney, M. T.; Cotton, J. M.; Richardson, P. J.; Shah, A. M. Postgrad. Med. J., 2001, 77, 4-10. [24] [5] Feldman, A. M.; McNamara, D. N. Engl. J. Med., 2000, 343, 1388-1398. Tipnis, S. R.; Hooper, N. M.; Hyde, R.; Karran, E.; Christie, G.; Turner, A. J. J. Biol. Chem., 2000, 275, 33238-43. [25] [6] Yazaki, Y.; Isobe, M.; Yamazaki, S.; Sekiguchi, M.; Usuda, N. Virchows Arch., 1998, 433, 161-6. Donoghue, M.; Hsieh, F.; Baronas, E.; Godbout, K.; Gosselin, M.; Stagliano, N.; Donovan, M.; Woolf, B.; Robison, K.; Jeyaseelan, R.; Breitbart, R. E.; Acton, S. Circ. Res., 2000, 87, E1-9. [7] Jacobson, D. L.; Gange, S. J.; Rose, N. R.; Graham, N. M. Clin. Immunol. Immunopathol., 1997, 84, 223-43. [26] [8] Anandasabapathy, S.; Frishman, W. H. J. Clin. Pharm., 1998, 38, 295-308. Mukoyama, M.; Nakajima, M.; Horiuchi, M.; Sasamura, H.; Pratt, R. E.; Dzau, V. J. J. Biol. Chem., 1993, 268, 24539-42. [27] Iwai, N.; Inagami, T. FEBS Lett., 1992, 298, 257-60. [9] Rossi, M. A.; Bestetti, R. B. Cardiology, 1995, 86, 1-7. [28] [10] Wenger, N. K.; Abelmann, W. H.; Roberts, W. C. In The Heart, J.W. Hurst; R.C. Schlant, eds.; McGraw Hill: New York, NY, 1990, pp. 1256-1277. Timmermans, P. B.; Wong, P. C.; Chiu, A. T.; Herblin, W. F.; Benfield, P.; Carini, D. J.; Lee, R. J.; Wexler, R. R.; Saye, J. A.; Smith, R. D. Pharmacol. Rev., 1993, 45, 20551. ACE and Myocarditis [29] Davis, G. K.; Roberts, D. H. Pharm. Therap., 1997, 75, 43-50. [30] Nio, Y.; Matsubara, H.; Murasawa, S.; Kanasaki, M.; Inada, M. J. Clin. Invest., 1995, 95, 46-54. [31] Ohkubo, N.; Matsubara, H.; Nozawa, Y.; Mori, Y.; Murasawa, S.; Kijima, K.; Maruyama, K.; Masaki, H.; Tsutumi, Y.; Shibazaki, Y.; Iwasaka, T.; Inada, M. Circulation, 1997, 96, 3954-62. [32] Lambert, C.; Massillon, Y.; Meloche, S. Circ. Res., 1995, 77, 1001-7. [33] Lambert, C.; Massillon, Y.; Meloche, S. In Mechanisms of Heart Failure, P.K. Singal; I.M.C. Dixon; R.E. Beamish; N. S. Dhalla, eds.; Kluwer Academic Publishers: Boston, MA, 1995, pp. 203-214. [34] Tsutsumi, Y.; Matsubara, H.; Ohkubo, N.; Mori, Y.; Nozawa, Y.; Murasawa, S.; Kijima, K.; Maruyama, K.; Masaki, H.; Moriguchi, Y.; Shibasaki, Y.; Kamihata, H.; Inada, M.; Iwasaka, T. Circ. Res., 1998, 83, 1035-46. Current Pharmaceutical Design, 2003, Vol. 9, No. 3 11 [48] Godsel, L. M.; Wang, K.; Schodin, B. A.; Leon, J. S.; Miller, S. D.; Engman, D. M. Circulation, 2001, 103, 1709-1714. [49] Wang, Y.; Afanasyeva, M.; Hill, S. L.; Kaya, Z.; Rose, N. R. J. Am. Coll. Cardiol., 2000, 36, 1992-9. [50] Smith, S. C.; Allen, P. M. Immunol. Ser., 1993, 59, 377386. [51] Smith, S. C.; Allen, P. M. Clin. Immunol. Immunopathol., 1993, 68, 100-106. [52] Bachmaier, K.; Pummerer, C.; Shahinian, A.; Ionescu, J.; Neu, N.; Mak, T. W.; Penninger, J. M. J. Immunol., 1996, 157, 1752-7. [53] Neu, N.; Pummerer, C.; Rieker, T.; Berger, P. Clin. Immunol. Immunopathol., 1993, 68, 107-10. [54] Penninger, J. M.; Neu, N.; Timms, E.; Wallace, V. A.; Koh, D. R.; Kishihara, K.; Pummerer, C.; Mak, T. W. J. Exp. Med., 1993, 178, 1837-42. [35] Suzuki, J.; Matsubara, H.; Urakami, M.; Inada, M. Circ. Res., 1993, 73, 439-47. [55] Malkiel, S.; Factor, S.; Diamond, B. J. Immunol., 1999, 163, 5265-5268. [36] Fujii, N.; Tanaka, M.; Ohnishi, J.; Yukawa, K.; Takimoto, E.; Shimada, S.; Naruse, M.; Sugiyama, F.; Yagami, K.; Murakami, K. Biochem. Biophys. Res. Commun., 1995, 212, 326-33. [56] Tanowitz, H. B.; Kirchhoff, L. V.; Simon, D.; Morris, S. A.; Weiss, L. M.; Wittner, M. Clin. Microbiol. Rev., 1992, 5, 400-419. [57] [37] Lopez, J. J.; Lorell, B. H.; Ingelfinger, J. R.; Weinberg, E. O.; Schunkert, H.; Diamant, D.; Tang, S. S. Am. J. Physiol., 1994, 267, H844-52. Yuan, Z.; Kishimoto, C.; Shioji, K.; Nakamura, H.; Yodoi, J.; Sasayama, S. Cardiovasc. Res., 2002, 55, 320-8. [58] Rezkalla, S.; Kloner, R. A.; Khatib, G.; Khatib, R. Circulation, 1990, 81, 1039-46. [38] Lee, Y. A.; Liang, C. S.; Lee, M. A.; Lindpaintner, K. Proc. Natl. Acad. Sci. USA, 1996, 93, 11035-40. [59] Rezkalla, S.; Kloner, R. A.; Khatib, G.; Khatib, R. Am. Heart J., 1990, 120, 1377-81. [39] Kawai, C. Circulation, 1999, 99, 1091-100. [60] [40] Neu, N.; Rose, N. R.; Beisel, K. W.; Herskowitz, A.; GurriGlass, G.; Craig, S. W. J. Immunol., 1987, 139, 36303636. Suzuki, H.; Matsumori, A.; Matoba, Y.; Kyu, B. S.; Tanaka, A.; Fujita, J.; Sasayama, S. J. Clin. Invest., 1993, 91, 2727-33. [61] [41] Caforio, A. L.; Goldman, J. H.; Haven, A. J.; Baig, K. M.; McKenna, W. J. Int. J. Cardiol., 1996, 54, 157-63. de Cavanagh, E. M.; Inserra, F.; Ferder, L.; Romano, L.; Ercole, L.; Fraga, C. G. FEBS Lett., 1995, 361, 22-4. [62] [42] Lauer, B.; Padberg, K.; Schultheiss, H. P.; Strauer, B. E. J. Am. Coll. Cardiol., 1994, 23, 146-53. Araki, M.; Kanda, T.; Imai, S.; Suzuki, T.; Murata, K.; Kobayashi, I. J. Cardiovasc. Pharmacol., 1995, 26, 61-5. [63] [43] Lauer, B.; Schannwell, M.; Kuhl, U.; Strauer, B.; Schultheiss, H. J. Am. Coll. Cardiol., 2000, 35, 11-18. Kanda, T.; Araki, M.; Nakano, M.; Imai, S.; Suzuki, T.; Murata, K.; Kobayashi, I. J. Pharm. Exp. Ther., 1995, 273, 955-8. [44] Goldman, J. H.; Keeling, P. J.; Warraich, R. S.; Baig, M. K.; Redwood, S. R.; Dalla Libera, L.; Sanderson, J. E.; Caforio, A. L.; McKenna, W. J. Br. Heart J., 1995, 74, 598-603. [64] Takada, H.; Kishimoto, C.; Hiraoka, Y.; Kurokawa, M.; Shiraki, K.; Sasayama, S. Am. J. Physiol., 1997, 272, H211-9. [65] [45] Neumann, D. A.; Burek, C. L.; Baughman, K. L.; Rose, N. R.; Herskowitz, A. J. Am. Coll. Cardiol., 1990, 16, 839846. Baba, T.; Kanda, T.; Kobayashi, I. Life Sci., 2000, 67, 587-97. [66] Tanaka, A.; Matsumori, A.; Wang, W.; Sasayama, S. Circulation, 1994, 90, 2051-5. [46] Latif, N.; Smith, J.; Dunn, M. J.; Yacoub, M. H.; Rose, M. L. Autoimmunity, 1994, 19, 99-104. [67] Gardner, P. L.; Mbuy, G. N.; Knabb, M. T. Life Sci., 1994, 55, 283-9. [47] Latif, N.; Baker, C. S.; Dunn, M. J.; Rose, M. L.; Brady, P.; Yacoub, M. H. J. Am. Coll. Cardiol., 1993, 22, 1378-84. [68] Rassi, A., Jr.; Rassi, A.; Little, W. C. Clin. Cardiol., 2000, 23, 883-9. 12 Current Pharmaceutical Design, 2003, Vol. 9, No. 3 [88] Clapperton, M.; McMurray, J.; Fisher, A. C.; Dargie, H. J. Br. J. Clin. Pharmacol., 1994, 38, 53-6. [89] Clozel, M.; Kuhn, H.; Hefti, F.; Baumgartner, H. R. Hypertension, 1991, 18, 132-41. [90] Bellabarba, G.; Davila, D. F.; Torres, A.; Donis, J. H.; Gonzalez, J. C.; Figueroa, O.; Vasquez, C. J.; Faddoul, M.; Khoury, A. Int. J. Cardiol., 1994, 47, 5-11. Raiden, S.; Giordano, M.; Andonegui, G.; Trevani, A. S.; Lopez, D. H.; Nahmod, V.; Geffner, J. R. J. Pharm. Exp. Ther., 1997, 281, 624-8. [91] Khoury, A. M.; Davila, D. F.; Bellabarba, G.; Donis, J. H.; Torres, A.; Lemorvan, C.; Hernandez, L.; Bishop, W. Int. J. Cardiol., 1996, 57, 21-9. Kupatt, C.; Habazettl, H.; Zahler, S.; Weber, C.; Becker, B. F.; Messmer, K.; Gerlach, E. Cardiovasc. Res., 1997, 36, 386-95. [92] Scharfstein, J.; Schmitz, V.; Morandi, V.; Capella, M. M.; Lima, A. P.; Morrot, A.; Juliano, L.; Muller-Esterl, W. J. Exp. Med., 2000, 192, 1289-300. Guba, M.; Steinbauer, M.; Buchner, M.; Frolich, D.; Farkas, S.; Jauch, K. W.; Anthuber, M. Shock, 2000, 13, 190-6. [93] Hoshida, S.; Yamashita, N.; Kawahara, K.; Kuzuya, T.; Hori, M. Circulation, 1999, 99, 434-40. [94] Jilma, B.; Li-Saw-Hee, F. L.; Wagner, O. F.; Beevers, D. G.; Lip, G. Y. Clin. Sci. (Lond.), 2002, 103, 131-6. [69] Batlouni, M.; Barretto, A. C.; Armaganijan, D.; Vichi, F. L.; Spritzer, N.; Simoes, R.; Hatab, S. A.; Nascimento, L. O. Arq. Bras. Cardiol., 1992, 58, 417-21. [70] Roberti, R. R.; Martinez, E. E.; Andrade, J. L.; Araujo, V. L.; Brito, F. S.; Portugal, O. P.; Horowitz, S. F. Eur. Heart J., 1992, 13, 966-70. [71] [72] [73] [74] Engman et al. Constantinescu, C. S.; Ventura, E.; Hilliard, B.; Rostami, A. Immunopharmacol. Immunotoxicol., 1995, 17, 47191. [75] Weinstock, J. V.; Boros, D. L. Gastroenterology, 1981, 81, 953-8. [95] Elferink, J. G.; de Koster, B. M. Naunyn Schmiedebergs Arch. Pharmacol., 1993, 347, 562-7. [76] Schrier, D. J.; Ripani, L. M.; Katzenstein, A. L.; Moore, V. L. J. Clin. Invest., 1982, 69, 651-7. [96] Elferink, J. G. Agents Actions, 1993, 38, C136-8. [97] [77] Deepe, G. S., Jr.; Taylor, C. L.; Srivastava, L.; Bullock, W. E. Infect. Immun., 1985, 48, 395-401. Strawn, W. B.; Gallagher, P. E.; Tallant, E. A.; Ganten, D.; Ferrario, C. M. J. Cardiovasc. Pharmacol., 1999, 33, 341-51. [78] Rodgers, K.; Xiong, S.; Espinoza, T.; Roda, N.; Maldonado, S.; diZerega, G. S. Clin. Diagn. Lab. Immunol., 2000, 7, 635-40. [98] Luvara, G.; Pueyo, M. E.; Philippe, M.; Mandet, C.; Savoie, F.; Henrion, D.; Michel, J. B. Arterioscler. Thromb. Vasc. Biol., 1998, 18, 1408-16. [79] Hisada, Y.; Sugaya, T.; Yamanouchi, M.; Uchida, H.; Fujimura, H.; Sakurai, H.; Fukamizu, A.; Murakami, K. J. Clin. Invest., 1999, 103, 627-35. [99] Mervaala, E. M.; Muller, D. N.; Park, J. K.; Schmidt, F.; Lohn, M.; Breu, V.; Dragun, D.; Ganten, D.; Haller, H.; Luft, F. C. Hypertension, 1999, 33, 389-95. [80] Ruiz-Ortega, M.; Lorenzo, O.; Egido, J. Kidney Int., 2000, 57, 2285-98. [100] Grafe, M.; Auch-Schwelk, W.; Zakrzewicz, A.; RegitzZagrosek, V.; Bartsch, P.; Graf, K.; Loebe, M.; Gaehtgens, P.; Fleck, E. Circ. Res., 1997, 81, 804-11. [81] Hernandez-Presa, M.; Bustos, C.; Ortego, M.; Tunon, J.; Renedo, G.; Ruiz-Ortega, M.; Egido, J. Circulation, 1997, 95, 1532-41. [101] Morrissey, J. J.; Klahr, S. Am. J. Physiol., 1998, 274, F580-6. [82] Hernandez-Presa, M. A.; Bustos, C.; Ortego, M.; Tunon, J.; Ortega, L.; Egido, J. Am. J. Pathol., 1998, 153, 182537. [102] Nataraj, C.; Oliverio, M. I.; Mannon, R. B.; Mannon, P. J.; Audoly, L. P.; Amuchastegui, C. S.; Ruiz, P.; Smithies, O.; Coffman, T. M. J. Clin. Invest., 1999, 104, 1693-701. [83] Soejima, H.; Ogawa, H.; Yasue, H.; Kaikita, K.; Takazoe, K.; Nishiyama, K.; Misumi, K.; Miyamoto, S.; Yoshimura, M.; Kugiyama, K.; Nakamura, S.; Tsuji, I. J. Am. Coll. Cardiol., 1999, 34, 983-8. [103] Kunert-Radek, J.; Stepien, H.; Komorowski, J.; Pawlikowski, M. Biochem. Biophys. Res. Commun., 1994, 198, 1034-9. [84] Kintscher, U.; Wakino, S.; Kim, S.; Fleck, E.; Hsueh, W. A.; Law, R. E. Hypertension, 2001, 37, 587-93. [85] Piqueras, L.; Kubes, P.; Alvarez, A.; O'Connor, E.; Issekutz, A. C.; Esplugues, J. V.; Sanz, M. J. Circulation, 2000, 102, 2118-23. [86] Alvarez, A.; Sanz, M. J. J. Leukoc. Biol., 2001, 70, 199206. [87] Raiden, S.; Pereyra, Y.; Nahmod, V.; Alvarez, C.; Castello, L.; Giordano, M.; Geffner, J. J. Leukoc. Biol., 2000, 68, 700-6. [104] Okamura, A.; Rakugi, H.; Ohishi, M.; Yanagitani, Y.; Takiuchi, S.; Moriguchi, K.; Fennessy, P. A.; Higaki, J.; Ogihara, T. J. Hypertens., 1999, 17, 537-45. [105] Chisi, J. E.; Wdzieczak-Bakala, J.; Thierry, J.; Briscoe, C. V.; Riches, A. C. Stem Cells, 1999, 17, 339-44. [106] Chisi, J. E.; Briscoe, C. V.; Ezan, E.; Genet, R.; Riches, A. C.; Wdzieczak-Bakala, J. Br. J. Haematol., 2000, 109, 563-70. [107] Simon, M. R.; Weinstock, J. V.; Morrell, R. M.; Roi, L. D.; Howard, L. M. Agents Actions, 1984, 15, 525-8. ACE and Myocarditis [108] Binderup, L.; Bramm, E.; Arrigoni-Martelli, E. Experientia, 1982, 38, 399-401. [109] Tarkowski, A.; Carlsten, H.; Herlitz, H.; Westberg, G. Agents Actions, 1990, 31, 96-101. [110] Yeung, J. H. Meth. Find. Exp. Clin. Pharm., 1994, 16, 163-72. [111] Johnsen, S. A.; Persson, I. B.; Aurell, M. Scand. J. Urol. Nephrol., 1997, 31, 81-8. [112] Nakamura, A.; Johns, E. J.; Imaizumi, A.; Yanagawa, Y.; Kohsaka, T. Cytokine, 1999, 11, 759-65. [113] Peeters, A. C.; Netea, M. G.; Kullberg, B. J.; Thien, T.; van der Meer, J. W. Immunology, 1998, 94, 376-9. [114] Gullestad, L.; Aukrust, P.; Ueland, T.; Espevik, T.; Yee, G.; Vagelos, R.; Froland, S. S.; Fowler, M. J. Am. Coll. Cardiol., 1999, 34, 2061-7. [115] Wei, G. C.; Sirois, M. G.; Qu, R.; Liu, P.; Rouleau, J. L. J. Cardiovasc. Pharmacol., 2002, 39, 842-50. [116] Schindler, R.; Dinarello, C. A.; Koch, K. M. Cytokine, 1995, 7, 526-33. [117] Odaka, C.; Mizuochi, T. Clin. Exp. Immunol., 2000, 121, 515-22. [118] Constantinescu, C. S.; Goodman, D. B.; Ventura, E. S. Immunolology letters, 1998, 62, 25-31. [119] Oben, J. A.; Wallace, G. R.; Chain, B. M.; Foreman, J. C. Immunology, 1989, 67, 328-32. [120] Hahn, A. W.; Jonas, U.; Buhler, F. R.; Resink, T. J. FEBS Lett., 1994, 347, 178-80. [121] Zhao, S. P.; Xie, X. M. Clin. Chim. Acta, 2001, 304, 8590. [122] Stenvinkel, P.; Andersson, P.; Wang, T.; Lindholm, B.; Bergstrom, J.; Palmblad, J.; Heimburger, O.; Cederholm, T. J. Int. Med., 1999, 246, 503-7. [123] Fukuzawa, M.; Satoh, J.; Sagara, M.; Muto, G.; Muto, Y.; Nishimura, S.; Miyaguchi, S.; Qiang, X. L.; Sakata, Y.; Nakazawa, T.; Ikehata, F.; Ohta, S.; Toyota, T. Immunopharmacology, 1997, 36, 49-55. [124] Kupfahl, C.; Pink, D.; Friedrich, K.; Zurbrugg, H. R.; Neuss, M.; Warnecke, C.; Fielitz, J.; Graf, K.; Fleck, E.; Regitz-Zagrosek, V. Cardiovasc. Res., 2000, 46, 463-75. [125] Shin, G. T.; Kim, S. J.; Ma, K. A.; Kim, H. S.; Kim, D. Am. J. Kidney Dis., 2000, 36, 894-902. Current Pharmaceutical Design, 2003, Vol. 9, No. 3 13 [126] Sharma, K.; Eltayeb, B. O.; McGowan, T. A.; Dunn, S. R.; Alzahabi, B.; Rohde, R.; Ziyadeh, F. N.; Lewis, E. J. Am. J. Kidney Dis., 1999, 34, 818-23. [127] Campistol, J. M.; Inigo, P.; Jimenez, W.; Lario, S.; Clesca, P. H.; Oppenheimer, F.; Rivera, F. Kidney Int., 1999, 56, 714-9. [128] Foris, G.; Dezso, B.; Medgyesi, G. A.; Fust, G. Immunology, 1983, 48, 529-35. [129] Lijnen, P.; Fagard, R.; Petrov, V. J. Hypertens., 1997, 15, 871-6. [130] Xie, L.; Chen, D.; Wu, D.; Wang, H. J. Human Hypertens., 1996, 10, 425-7. [131] Wawrocka-Pawlak, M.; Dabrowski, R. Inflamm. Res., 1999, 48, 262-4. [132] Miselis, J.; Siminiak, T.; Wysocki, H. J. Human Hypertens., 1994, 8, 565-9. [133] Kranzhofer, R.; Browatzki, M.; Schmidt, J.; Kubler, W. Biochem. Biophys. Res. Commun., 1999, 257, 826-8. [134] Pueyo, M. E.; Gonzalez, W.; Nicoletti, A.; Savoie, F.; Arnal, J. F.; Michel, J. B. Arterioscler. Thromb. Vasc. Biol., 2000, 20, 645-51. [135] Muller, D. N.; Mervaala, E. M.; Schmidt, F.; Park, J. K.; Dechend, R.; Genersch, E.; Breu, V.; Loffler, B. M.; Ganten, D.; Schneider, W.; Haller, H.; Luft, F. C. Hypertension, 2000, 36, 282-90. [136] Simon, M. R.; Kamlay, M. T.; Desai, S. G.; Majumdar, A. P. Biochem. Med. Metab. Biol., 1991, 45, 48-55. [137] Ruiz-Gines, J. A.; Perez-Caballero, C.; O'Valle, F.; Rodriguez-Puyol, M.; Rodriguez-Puyol, D. Eur. J. Pharmacol., 1997, 336, 251-256. [138] Napoleone, E.; Di Santo, A.; Camera, M.; Tremoli, E.; Lorenzet, R. Circ. Res., 2000, 86, 139-43. [139] Bestetti, R. B.; Dalbo, C. M.; Freitas, O. C.; Teno, L. A.; Castilho, O. T.; Oliveira, J. S. Cardiology, 1994, 84, 2617. [140] Szajnbok, F. E.; Barretto, A. C.; Mady, C.; Parga Filho, J.; Gruppi, C.; Alfieri, R. G.; da Luz, P. L.; Pileggi, F. Arq. Bras. Cardiol., 1993, 60, 273-8.