* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Somatotopic Mapping of the Subthalamic Nucleus and the Effects of
Survey
Document related concepts
Transcript
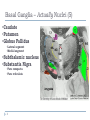
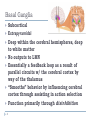
Advances in the Treatment of Parkinson’s Disease Rennie U. Jacobs, MHS, LOTR, CHT What do you know about Parkinson’s Disease? What are the cardinal symptoms? Where is the damage in the brain? What neurotransmitter is lacking? How quickly does it progress? What type of neurologist/neurosurgeon treats PD? What can therapist’s do to help? Basal Ganglia – Actually Nuclei (5) •Caudate •Putamen •Globus Pallidus •Lateral segment •Medial segment •Subthalamic nucleus •Substantia Nigra •Pars compacta •Pars reticulata 3 Basal Ganglia Subcortical Extrapyramidal Deep within the cerebral hemispheres, deep to white matter No outputs to LMN Essentially a feedback loop as a result of parallel circuits w/ the cerebral cortex by way of the thalamus “Smooths” behavior by influencing cerebral cortex through assisting in action selection Function primarily through disinhibition 4 Neurotransmitter Specificity Efferent Axons GABA (-) GABA (-) GABA (-) GABA (-) Dopamine (+) GABA (-) Glutamate (+) 5 Nolte, 19.1 Damage to the Basal Ganglia Movement disorders: Hypokinetic Parkinson’s disease (loss of dopanergic neurons in SNc) Parkinsonisms Hyperkinetic 6 Hemiballism (loss of subthalamic nucleus) Tics/Tourette’s (smaller BG on one side, usually left) Huntington’s Chorea (atrophy of caudate and putamen) Tardive dyskinesia (overmedication with antipsychotic/antidopanergic drugs) Direct vs. Indirect Pathways 7 DIRECT PATHWAY: facilitates behavior Net effect: Increased activity of the thalamus and subsequent excitation of cerebral cortex 8 INDIRECT PATHWAY: suppresses behavior Net effect: Decreased activity of the thalamus and subsequent suppression of cortical activity 9 Parkinson’s Disease 10 Parkinson’s Disease (PD) 11 Progressive and debilitating affects 1 in 100 people (1%) over age 60 It is suggested that at least 1 million people in the US suffer from PD, mean age onset of symptoms is 55 First characterized extensively by James Parkinson in 1817 Primarily ideopathic Possible causes of Parkinsonism: genetics (younger patients), environmental toxins (MPTP), and head trauma (pugilistic Parkinson disease) Pathophysiology 12 Loss of dopaminergic neurons in substantia nigra compacta Decreased nigrostriatal projections Increased activity of “indirect pathway” http://neurophilosophy.wordpress.com/2006/07/05/dopamine-receptor-agonist-stimulates-neurogenesis-leads-tofunctional-recovery-in-parkinsonian-rats/ PD Symptoms – Vary in severity Cardinal symptoms: 13 Rigidity Resting tremors Akinesia and Bradykinesia Loss of postural reflexes > instability Diagnosis of PD No tests available to diagnose PD Symptom presentation are hallmarks of disease 14 2 or more Often begin unilaterally then progress to bilateral, asymmetry common Responsiveness to Parkinson’s medications used as evidence of disease Can be misdiagnosed as medication induced PD, essential tremor, normal pressure hydrocephalus, progressive supranuclear palsy Unified Parkinson’s Disease Rating Scale UPDRS Unified and accepted mechanism for following the progression of PD Part I - Mentation, behavior, and mood Part II - Activities of daily life (ADLs) Part III – Motor Part IV – Hoenh and Yahr scoring of severity (0-5) Part V - Schwab & England Activities of Daily Living Scale “OFF – ON Challenge” 15 Treatment of PD Medications Dopamine Replacement Therapies Dopamaine agonists MAO-B inhibitors – prolong effects of dopamine Surgeries Ablative surgery Pallidotomy (GPi) Thalamotomy (ventral intermediate nucleus) Deep brain stimulation Gpi STN – sensorimotor portion (dorsolateral) 16 Deep Brain Stimulation (DBS) high-frequency electrical stimulation of specific brain targets Shuts down activity at the site Mimics the effect of a lesion without the need for destroying brain tissue Advantages: 17 Doesn’t require making a destructive lesion Can be performed bilaterally with relative safety Stimulation parameters can be adjusted postoperatively to improve efficacy, reduce adverse effects, and to adapt to the progression of the disease Reversible, therefore doesn’t preclude the use of possible future therapies for PD STN General Topography – DBS Target 18 Challenges to DBS Direct visualization of nuclei difficult Cho et al. found superiority of using 7-T MR imaging (compared to 1.5- and 3-T), enabling authors to visualize STN and GPi on T2-weighted images. Lack of visualization makes targeting nucleus difficult Once in nucleus, difficult to determine where you are and what the ideal placement of the electrode will be 19 How Can We Utilize Neurophysiological Data to Better Describe STN? 20 Is There a Homunculus? Presence of a sensorimotor map would better guide surgical procedures, theoretically resulting in better outcomes Primate studies have described the presence of a somatopic map in the motor territory of the primate STN Several groups have performed human studies and developed a proposed map, however there is opportunity and need for more rigorous, exact correlations statistically. 21 How Can We Utilize Neurophysiological Data to Better Describe STN? Specific Aim: Test the hypothesis that that the human STN has an organization similar to that in normal monkey, and that with disease progression, surviving cells develop enlarging receptive fields as a compensatory mechanism with resultant “blurring” of regional distinctions within the nucleus. Significance: The suggestion of a homunculus has guided surgical procedures in the past. This current study has great potential in contributing fundamental understanding of the individual variation in the mapping characteristics between patients as the disease progresses. We aim to clear an area of ambiguity in the neurosurgery literature by using statistical analysis to describe the sensorimotor representation in the human STN of patients with PD, as well as determine if there is a scale of despecification with the progression of the disease. Results from this study may have immediate effect on surgical practices worldwide. 22 Human Studies 23 Romanelli P; Heit G; Hill BC; Kraus A; Hastie T; Brontë-Stewart HM (2004) Microelectrode Recording Revealing a Somatotopic Body Map in the Subthalamic Nucleus in Humans with Parkinson Disease Journal of Neurosurgery, Vol. 100 (4), pp. 611-8. Retrospective review of intraoperative electrophysiological data Used velocity sensors to record joint motions Although some single neuronal responses were observed, recordings were primarily from more than one cell, or “unit recordings” Position and type of each unit’s response was recorded on scaled graph paper > transcribed on clear film Film overlaid on scaled parasagittal plane map of target region to compare the physiological data with atlas This constituted their electrophysiological spatial map Analysis of the spatial relationships b/w STN units w/ UE and LE somatosensory driving showed leg-related units located medially and ventrally to arm-related units 24 Theodosopoulos PV; Marks WJ Jr; Christine C; Starr PA (2003) Locations of Movement-Related Cells in the Human Subthalamic Nucleus in Parkinson’s Disease Movement Disorders, Vol. 18(7), 791-798. Utilized single microelectrode penetrations (range 1-7, mean 3.8) Cells were considered movement-related if they exhibited modulation of the cell discharge during passive movements 15 procedures analyzed Audible alterations synchronous with the movement Presence of at least seven movement-related cells distributed among at least three tracks Selected microelectrode tracks were localized on the STN referencing the Schaltenbrand and Wahren human brain atlas and post-op visualization of the DBS lead MRI Statisical analyses were performed to determine if arm-related and leg-related cells had different distributions Proportion of leg cells over arm cells in medial half of nucleus (Fischer’s twotailed exact test) 25 Rodriguez-Oroz MC; Rodriguez M; Guridi J; Mewes K; Chockkman V; Vitek J; DeLong MR; Obeso JA. (2001) The Subthalamic Nucleus in Parkinson’s Disease: Somatotopic Organization and Physiological Characteristics Brain,Vol. 124, 1777-1790. EMG activity of tibialis anterior and extensor carpi radialis recorded simultanteously with neuronal recordings Neurons with sensorimotor responses noted intra-operatively and relative position in the tract was noted using x and z coordinates (mediolateral (x) and dorsoventral (z)) DBS lead placement on post-op MRI was used as objective reference From medial to lateral, proportion of neurons responding to movement of leg decreased, while arm increased 77% of arm cells were in lateral planes (>12mm) 51% of leg cells were medial to 12mm plane 26 Abosch A; Hutchinson WD; Saint-Cyr JA; Dostrovsky JO; Lozano AM. (2002) Movement-Related Neurons of the Subthalamic Nucleus in Patients with Parkinson Disease J Neurosurg, Vol 97, 1167-1172. Intraoperatively, cells were tested for response to passive motion Reproducible , audible change in firing rate Recorded and time-locked to the movement of the specific joint Electrophysiological data retrospectively collected from microelectrode recordings Analysis of receptive fields obtained along individual electrode tracks through the STN did not reveal any consistent pattern of somatotopic organization 27 Experimental Design 28 IRB Approval, Patient Selection, Consent IRB approval received 9/2009 (#7320) Patients undergoing DBS to STN for relief of symptoms related to PD meet criteria for study To date, 6 patients enrolled in study and properly consented 29 4 bilateral 2 unilateral Microelectrode Data MicroGuide™ System (Alpha Omega Company, Alpharetta, Georgia, US) Microelectrodes are connected to the recording tower, which collects and stores data from 1-5 channels 30 Measurement of Joint Position – Electronic Goniometers PASCO® PASPORT goniometer sensors and probes PASCO ® PowerLink Collects angle, velocity, acceleration at each joint Capable of writing data to text files for further analyses Range Accuracy <1% of measurement Sampling Rate 0 – 340° (-170° to +170°) 100Hz All needed equipment 31 ~$1000 Data Collection - Methods Patient is brought to OR Electronic goniometers are attached to patient and input to an instrument hub (PASCO Powerlink, PASCO, Roseville, California, US) 32 Contralateral elbow, hip, and knee (e.g. DBS to right brain, goniometers on left limbs) Data Collection - Methods A digital adapter is wired from a Data Acquisition Board (DAQ) digital output to the PASCO Powerlink Microelectrode activity wired directly from splitters sending duplicate data to the Micro-Guide System an the DAQ board Both PASCO Powerlink and DAQ board are connected to a single research laptop via USB 33 Data Collection - Methods Neurosurgeon preps patient for microelectrode recording Software capable of collecting joint motion data and microelectrode data are started 34 Data Collection - Methods Neurophysiologist begins advancing the microelectrodes through the brain Data are taken beginning 15 mm above what is expected to be the bottom of the STN based on trajectory planning At each 0.5 mm, the research assistant passively moves the patient’s elbow, hip, and knee separately in both flexion and extension 35 Data Collection - Methods Recordings are continued in 0.5 mm increments until the cellular activity indicates that all 5 microelectrodes have passed completely through the STN Response more indicative of substantia nigra or white matter Software is stopped, and all data are saved for post-surgery conversion 36 Research Equipment and Flow of Data 37 Establishment of a Single Chronological Frame of Reference - ClockSynch Visual Basic .NET (VB.NET) program Created by Tyler Moore, BS, LSUHSC School of Medicine Collects microelectrode data at 25 kHz via a Data Acquisition Board (USB-6212 DAQ Board, National Instruments, Austin, TX) Sends random digital pulses to PASCO software via PASCO Powerlink Data is stored in binary file during surgery Post-operatively, ClockSynch capable of converting binary files to tab-delimited text files 38 Preliminary Results 39 Preliminary Results - Spatial Atlas of STN Depth of 10.5 Depth of 3.5 Depth of 1.5 40 Preliminary Results - Spatial Atlas of STN Depth of 10.5 Depth of 3.5 41 Statistical Analysis – Sensorimotor Representation of STN MATLAB R2009a Ted Weyand, PhD, LSUHSC Data files were reduced to a more maneagable size by converting ascii files to 16 bit binary format Data were then subject to a Gaussian filter to smooth the data Take the absolute value of all points Averaged every 5 data points to begin smoothing filter Data divided into epochs that are considered signal if exceeding 2.5 sdu Resulting data then subject to Boolean logic 42 >threshold = 1 <threshold = 0 Statistical Analysis Final product: Data reduced and displayed at a lower resolution (10Hz) to match goniometer data Excess of 2.5 sdus considered an “event” Greater than 5-10 degrees of change of motion considered “event”, event expanded to cover 500 ms Chi-square analysis done to test for goodness of fit between cell activity and goniometer data. Results used to generate a 3-dimensional map of motorrelated activity in space 43 3-Dimensional Map 44 Future Research 45 Future Research Spatial atlas – 46 This spatial matrix can be further developed in such a way that a trainee can select target and entry points of their own choosing and perform virtual microelectrode recording along the resulting trajectory, allowing for practice outside of the clinical and time constraints of the operating room and improved learning. This simulation tool has implications to assist surgeons in trajectory mapping, surgical procedure, and discrimination of cellular activity. Future Research Sensorimotor Representation – 47 ideally would like single cell analysis but signal:noise ratio typically not high enough Looking at cross-correlation across electrode sites Storage of activity patterns for analysis to better understand potential prognosis, with a better understanding of how lack of dopamine alters endogenous activity patterns. Therapist’s Role 48 Therapist’s Role Advocate for your clients pre-operatively Assist w/ client education re: disease process, as well as medical management options Perform outcome measures to determine gains made from surgery 49 What do you know about Parkinson’s Disease? What are the cardinal symptoms? Where is the damage in the brain? What neurotransmitter is lacking? How quickly does it progress? What type of neurologist/neurosurgeon treats PD? What can therapist’s do to help? Questions???? 51 Thank You! 52