* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Anterior and Middle Deltoid Are Functionally Critical Targets for
Survey
Document related concepts
Transcript
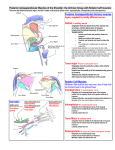
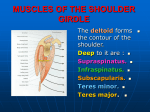
ANTERIOR AND MIDDLE DELTOID ARE FUNCTIONALLY CRITICAL TARGETS FOR NERVE TRANSFER FOLLOWING C5-C6 ROOT AVULSION INJURY 1,2 1 Dustin L. Crouch, 3Johannes Plate, 3Zhongyu Li and 1,2Katherine R. Saul Virginia Tech-Wake Forest School of Biomedical Engineering and Sciences, Winston-Salem, NC, USA 2 Department of Biomedical Engineering, Wake Forest School of Medicine, Winston-Salem, NC, USA 3 Department of Orthopaedic Surgery, Wake Forest School of Medicine, Winston-Salem, NC, USA email: [email protected], web: http://www.sbes.vt.edu/kholzbau/MoBL/ INTRODUCTION The deltoid and teres minor muscles, which are innervated by the axillary nerve, are paralyzed following brachial plexus avulsion injury of the C5 and C6 nerve roots. A nerve transfer to the axillary nerve is commonly performed to restore abduction strength. During this procedure, the radial nerve branch innervating the long head of the triceps is transferred to the anterior branch of the axillary nerve. Depending on the axillary nerve anatomy, either the entire deltoid or the anterior and middle compartments of the deltoid are restored, while teres minor remains paralyzed [1]. While this technique increases the likelihood that useful abduction strength is recovered, it is unclear whether overall function can be improved if teres minor and posterior deltoid are restored in addition to the anterior and middle deltoid compartments. Therefore, we used a musculoskeletal model to assess the biomechanical roles of teres minor and the three deltoid compartments in the context of isometric strength and shoulder movement. METHODS We used a computational musculoskeletal model of the upper limb [2] implemented for dynamic simulation [3] in OpenSim [4]. The model was simplified to represent 5 degrees of freedom at the shoulder, elbow, and forearm, and included 32 muscle compartments crossing the shoulder and elbow joints. Four scenarios were developed in which muscles were selectively paralyzed to investigate the biomechanical role of teres minor and the anterior, middle, and posterior deltoid muscle compartments (Table 1). Muscles were assumed to be unimpaired unless otherwise indicated. Paralyzed muscles were allowed to generate passive muscle forces only. Table 1: Scenario descriptions Paralyzed muscles and muscle Scenario compartments* unimpaired none 1 teres minor 2 teres minor, posterior deltoid teres minor, deltoid (all compartments) *Muscles not listed were assumed to be unimpaired. 3 Maximum isometric abduction and external rotation strength were calculated for each scenario by summing the maximum isometric joint moment that each muscle could generate in a given posture. Abduction strength was calculated at 45° shoulder elevation in the frontal plane with the elbow fully extended. External shoulder rotation strength was calculated with the arm and forearm in neutral posture and the elbow flexed to 90°. To understand how differences in shoulder strength among scenarios may affect movement performance, we conducted dynamic simulations of abduction from 0° to 90° in the frontal plane with the elbow extended and the forearm in a neutral posture. We used a computed muscle control (CMC) algorithm to compute the muscle activations required to track the movement as accurately as possible while minimizing a cost function related to muscle effort [5]. Shoulder elevation and flexion angles were evaluated to determine whether each scenario could abduct the shoulder to 90° and maintain neutral shoulder flexion. RESULTS AND DISCUSSION The largest differences in shoulder strength were observed for abduction (Fig.1). Compared to the unimpaired scenario, isometric abduction moment when both teres minor and posterior deltoid were paralyzed (scenario 2) was only 4.2% lower, while isometric abduction moment when teres minor and all deltoid compartments were paralyzed (scenario 3) was 62.0% lower. External rotation moment decreased only as much as 16.1% when teres minor and deltoid were paralyzed. These results suggest that teres minor and posterior deltoid contribute less to shoulder strength than do the anterior and middle deltoid compartments. Figure 2: Scenario 3 exhibited the least accuracy when attempting to simulate the abduction movement. Joint angles of scenario 1 and the unimpaired scenario are coincident. CONCLUSIONS Figure 1: Maximum isometric shoulder joint moments were similar when the anterior and middle deltoid compartments were not paralyzed (unimpaired, scenario 1, and scenario 2). Scenarios in which the anterior and middle deltoid compartments were not paralyzed (unimpaired, scenario 1, and scenario 2) could simulate the abduction movement to within 4° of the desired shoulder elevation angle with less than 4° of anterior flexion (Fig.2). However, when the entire deltoid muscle was paralyzed (scenario 3), the model was least capable among all scenarios of accurately simulating the abduction movement trajectory. Therefore, paralysis of the posterior deltoid and teres minor had relatively little effect on the ability of scenarios 1 and 2 to track the abduction movement. Based on the findings of this study, the anterior and middle deltoid compartments contribute most to shoulder strength and movement ability, compared to teres minor and posterior deltoid. Therefore, reinnervating teres minor and posterior deltoid may yield little additional functional benefit. Understanding the biomechanical consequences of axillary nerve transfers is important for surgical planning and predicting functional outcomes in clinical practice, particularly when multiple orthopaedic procedures are indicated. REFERENCES 1. Leechavengvongs S, et al. J Hand Surg, 28A(4), 633- 638, 2003. 2. Holzbaur KRS, et al. Ann Biomed Eng 33, 829-840. 3. Daly M, et al. ASB Conf Proceedings, State College, PA, 2009. 4. Delp SL, et al. IEEE Trans Biomed Eng 54, 19401950. 5. Thelen DG, Anderson FC. J Biomech 39, 1107-1115. ACKNOWLEDGEMENTS This work was supported by the National Institutes of Health (NIH 5R24HD050821-02) and the Wake Forest School of Medicine.