* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Diuretics
Discovery and development of direct Xa inhibitors wikipedia , lookup
Discovery and development of non-nucleoside reverse-transcriptase inhibitors wikipedia , lookup
Environmental impact of pharmaceuticals and personal care products wikipedia , lookup
Discovery and development of angiotensin receptor blockers wikipedia , lookup
Pharmacognosy wikipedia , lookup
Discovery and development of neuraminidase inhibitors wikipedia , lookup
Drug discovery wikipedia , lookup
Drug interaction wikipedia , lookup
Discovery and development of integrase inhibitors wikipedia , lookup
Psychopharmacology wikipedia , lookup
Discovery and development of ACE inhibitors wikipedia , lookup
Discovery and development of proton pump inhibitors wikipedia , lookup
Oral rehydration therapy wikipedia , lookup
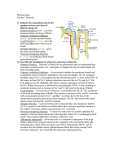
Introduction Diuretics are chemicals that increase the rate of urine formation. By increasing the urine flow rate, diuretic usage leads to increased excretion of electrolytes (especially sodium and chloride ions) and water from the body without affecting protein, vitamin, glucose, or amino acid reabsorption. These pharmacological properties have led to the use of diuretics in the treatment of edematous conditions resulting from a variety of causes (e.g., congestive heart failure, nephrotic syndrome, and chronic liver disease) and in the management of hyper tension. Diuretic drugs also are useful as the sole agent or as adjunct therapy in the treatment of a wide range of clinical conditions, including hypercalcemia, diabetes insipidus, acute mountain sickness, primary hyperaldosteronism, and glaucoma. Normal Physiology of urine formation Normal Physiology of Urine Formation Two important functions of the kidney are 1) to maintain a homeostatic balance of electrolytes and water and 2) to excrete water -soluble end products of metabolism. The kidney accomplishes these functions through the formation of urine by the nephrons. Each kidney contains approximately 1 million nephrons and is capable of forming urine independently. The nephrons are composed of a specialized capillary bed called the glomerulus and a long tubule divided anatomically and functionally into the proximal tubule, loop of Henle, and distal tubule. Each component of the nephron contributes to the normal functions of the kidney in a unique manner; thus, all are targets for different classes of diuretic agents. Urine formation begins with the filtration of blood at the glomerulus. Approximately 1,200 mL of blood per minute flows through both kidneys and reaches the nephron by way of afferent arterioles. Approximately 20% of the blood entering the glomerulus is filtered into Bowman's capsule to form the glomerular filtrate. The glomerular filtrate is composed of blood components with a molecular weight less than that of albumin (~69,000 daltons) and not bound to plasma proteins. The glomerular fil tration rate (GFR) averages 125 mL/min in humans but can vary widely even in normal functional states. The glomerular filtrate leaves the Bowman's capsule and enters the proximal convoluted tubule (S1, S2 segments, Fig 27.1), where the majority (50–60%) of filtered sodium is reabsorbed osmotically. Sodium reabsorption is coupled electrogenetically with the reabsorption of glucose, phosphate, and amino acids and nonelectrogenetically with bicarbonate reabsorption. Glucose and amino acids are completely reabsorbed in this portion of the nephron, whereas phosphate reabsorption is between 80 and 90% complete. The early proximal convoluted tubule also is the primary site of bicarbonate reabsorption (80–90%) , a process that is mainly sodium dependent and coupled to hydrogen ion secretion. The reabsorption of sodium and bicarbonate is facilitated by the enzyme carbonic anhydrase, which is present in proximal tubular cells and catalyzes the formation of carbonic acid from water and carbon dioxide. The carbonic acid provides the hydrogen ion, which drives the reabsorption of sodium bicarbonate. Chloride ions are reabsorbed passively in the proximal tubule, where they follow actively transported sodium ions into tubular cells. Clinical significance Diuretics have a variety of uses. Thiazide diuretics may be used either alone or in combination with other pharmacotherapy for the treatment of hyper tension. Loop diuretics can provide immediate diuresis and are used for heart failure and in lieu of thiazides in patients with compromised renal function. In addition to more traditional uses, certain potassium-sparing diuretics provide added benefit to other pharmacotherapy in patients with primary hyperaldosteronism, heart failure, or post–acute myocardial infarction. Carbonic anhydrase inhibitors have limited use for diuresis; however, they may be used to reduce intraocular pressure and treat acute mountain sickness. The reabsorption of electrolytes and water also occurs isosmotically in the proximal straight tubule or pars recta (S3 segment, Fig. 27.1) . By the end of the straight segment, between 65 and 70% of water and sodium, chloride, and calcium ions; 80 to 90% of bicarbonate and phosphate; and essentially 100% of glucose, amino acids, vitamins, and protein have been reabsorbed from the glomerular filtrate. The proximal tubule also is the site for active secretion of weakly acidic and weakly basic organic compounds. Thus, many of the diuretics can enter luminal fluid not only by filtration at the glomerulus but also by active secretion. The descending limb of the loop of Henle is impermeable to ions, but water can freely move from the luminal fluid into the surrounding medullary interstitium, where the higher osmolality draws water into the interstitial space and concentrates luminal fluid. Luminal fluid continues to concentrate as it descends to the deepest portion of the loop of Henle, where the fluid becomes the most concentrated. The hyper tonic luminal fluid next enters the waterimpermeable, thick ascending limb of the loop of Henle. In this segment of the nephron, approximately 20 to 25% of the filtered sodium and chloride ions are reabsorbed via a cotransport system (Na+/K+/2Cl -) on the luminal membrane. Reabsorption of sodium and chloride in the medullary portion of the thick ascending limb is important for maintaining the medullary interstitial concentration gradient. Reabsorption of sodium chloride in the cortical component of the thick ascending limb of the loop of Henle and the early distal convoluted tubule contributes to urinary dilution, and as a result, these two nephron sections sometimes are called the cortical diluting segment of the nephron. Luminal fluid leaving the early distal tubule next passes through the late distal tubule and cortical collecting tubule (collecting duct) , where sodium is reabsorbed in exchange for hydrogen and potassium ions. This process is partially controlled by mineralocorticoids (e.g., aldosterone) and accounts for the reabsorption of between 2 and 3% of filtered sodium ions. Although the reabsorption of sodium ions from these segments of the nephron is not large, this sodium/potassium/hydrogen ion exchange system determines the final acidity and potassium content of urine. Several factors, however, can influence the activity of this exchange system, including the amount of sodium ions delivered to these segments, the status of the acid-base balance in the body, and the levels of circulating aldosterone. Normal Regulation of Urine Formation The body contains several control mechanisms that regulate the volume and contents of urine. These systems are activated by changes in solute or water content of the body, by changes in systemic or renal blood pressure, and by a variety of other stimuli. Activation of one or more of these systems by diuretic drugs can modify the effectiveness of these drugs to produce their therapeutic response and may require additional therapeutic measures to ensure a maximal response. The kidney has the ability to respond to changes in the GFR through the action of specialized distal tubular epithelial cells called the macula densa. These cells are in close contact with the glomerular apparatus of the same nephron and detect changes in the rate of urine flow and luminal sodium chloride concentration. An increase in the urine flow rate at this site (as can occur with the use of some diuretics) activates the macula densa cells to communicate with the granular cells and vascular segments of the juxtaglomerular apparatus. Stimulation of the juxtaglomerular apparatus causes renin to be released, which leads to the formation of angiotensin II and subsequent renal vasoconstriction. Renal vasoconstriction leads to a decrease in GFR and, possibly, a decrease in the effectiveness of the diuretic. Renin release also can be stimulated by factors other than diuretics, including decreased renal per fusion pressure, increased sympathetic tone, and decreased blood volume. Another important regulatory mechanism for urine formation is antidiuretic hormone (ADH), also known as vasopressin, which is released from the posterior pituitary in response to reduced blood pressure and elevated plasma osmolality. In the kidney, ADH acts on the collecting tubule to increase water permeability and reabsorption. As a result, the urine becomes more concentrated, and water is conserved in the presence of ADH. Disease States The diuretic drugs are used primarily to treat two medically important conditions, edema and hypertension. Both conditions are common, although some patients exhibit refractory disease states that require additional modification of the drug regimen to include alternative diuretics or addition of nondiuretic drugs. Diuretic drugs may be administered acutely or chronically to treat edematous states. When immediate action to reduce edema (e.g., acute pulmonary edema) is needed, intravenous administration of a loop diuretic often is the approach of choice. Thiazide or loop diuretics normally are administered orally to treat nonemergency edematous states. The magnitude of the diuretic response is directly proportional to the amount of edema fluid that is present. As the volume of edema decreases, so does the magnitude of the diuretic response with each dose. If concern exists about diuretic-induced hypokalemia developing, then a potassium supplement or potassium-sparing diuretic may be added to the drug regimen. The development of hypokalemia is particularly important for patients with congestive heart failure who also are taking cardiac glycosides, such as digitalis. Digitalis has a narrow therapeutic index, and developing hypokalemia can potentiate digitalis- induced cardiac effects with potentially fatal results. Diuretic drugs (thiazide and loop diuretics) are administered orally to help control blood pressure in the treatment of hyper tension. Diuretics often are the first drugs used to treat hyper tension, and they also may be added to other drug therapies used to control blood pressure with beneficial effects. Structure Classification The diuretics currently in use today are classified by their chemical class (thiazides) , mechanism of action (carbonic anhydrase inhibitors and osmotics), site of action (loop diuretics), or effects on urine contents (potassium-sparing diuretics) . These drugs vary widely in their efficacy ( i.e., their ability to increase the rate of urine formation) and their site of action within the nephron. Efficacy often is measured as the ability of the diuretic to increase the excretion of sodium ions filtered at the glomerulus (i.e., the filtered load of sodium) and should not be confused with potency, which is the amount of the diuretic required to produce a specific diuretic response. Efficacy is determined, in part, by the site of action of the diuretic. Drugs (e.g., carbonic anhydrase inhibitors) that act primarily on the proximal convoluted tubule to induce diuresis are weak diuretics because of the ability of the nephron to reabsorb a significant portion of the luminal contents in latter portions of the nephron. Likewise, drugs (potassium-sparing diuretics) that act at the more distal segments of the nephron are weak diuretics, because most of the glomerular filtrate has already been reabsorbed in the proximal tubule and ascending limb of the loop of Henle before reaching the distal tubule. Thus, the most efficacious diuretics discovered so far, the high-ceiling or loop diuretics, interfere with sodium chloride reabsorption at the ascending limb of the loop of Henle, which is situated after the proximal tubule but before the distal portions of the nephron and collecting tubule. Classification of diuretics 1. Mercurials e.g. Mersalyl, Mercurophylline 2. Thiazide (Benzothiaziazines) e.g. Chlorthiazide, Hydrochlorthiazide, Benzthiazide 3. Sulphonamides e.g.Carbonic anhydrase inhibitors (Acetazolamide) 4. Water and Osmotic agents e.g. Water, Mannitol, Urea, Isosorbide 5. Sulphamyl Benzoic acid derivatives e.g. Furosimide, Clopamide 6. Endocrine antagonists e.g. Spironolactone, aldosterone, cortisone 7. Purines and related compounds e.g. caffeine, theophylline 8. Acidifying salts e.g. NH4Cl, NH4NO2 9. Phenoxyacetic acids e.g. Ethacrynic acid Carbonic Anhydrase Inhibitors Mechanism of Action In 1937, it was proposed that the normal acidificat ion of urine was caused by secretion of hydrogen ions by the tubular cells of the kidney. These ions were provided by the action of the enzyme carbonic anhydrase, which catalyzes the formation of carbonic acid (H2CO3) from carbon dioxide and water . It also was observed that sulfanilamide rendered the urine of dogs alkaline because of the inhibition of carbonic anhydrase. This inhibition of carbonic anhydrase resulted in a lesser exchange of hydrogen ions for sodium ions in the kidney tubule. Sodium ions, along with bicarbonate ions, and associated water molecules were then excreted, and a diuretic effect was noted. The large doses required and the side effects of sulfanilamide prompted a search for more effective carbonic anhydrase inhibitors as diuretic drugs. It was soon learned that the sulfonamide portion of an active diuretic molecule could not be monosubstituted or disubstituted. It was reasoned that a more acidic sulfonamide would bind more tightly to the carbonic anhydrase enzyme. Synthesis of more acidic sulfonamides produced compounds more than 2,500- fold more active than sulfanilamide. Acetazolamide was introduced in 1953 as an orally effective diuretic drug. Before that time, the organic mercurials, which commonly required intramuscular injection, were the principal diuretics available. Carbonic anhydrase inhibitors induce diuresis by inhibiting the formation of carbonic acid within proximal (proximal convoluted tubule; S2) and distal tubular cells to limit the number of hydrogen ions available to promote sodium reabsorption. For a diuretic response to be observed, more than 99% of the carbonic anhydrase must be inhibited. Although carbonic anhydrase activity in the proximal tubule regulates the reabsorption of approximately 20 to 25% of the filtered load of sodium, the carbonic anhydrase inhibitors are not highly efficacious diuretics. An increased excretion of only 2 to 5% of the filtered load of sodium is seen with carbonic anhydrase inhibitors because of increased reabsorption of sodium ions by the ascending limb of the loop of Henle and more distal nephron segments. Therapeutic Applications With prolonged use of the carbonic anhydrase inhibitor diuretics, the urine becomes more alkaline, and the blood becomes more acidic. When acidosis occurs, the carbonic anhydrase inhibitors lose their effectiveness as diuretics. They remain ineffective until normal acid-base balance in the body has been regained. For this reason, this class of compounds is limited in its diuretic use. Today, they are most commonly used in the treatment of glaucoma, in which they reduce the rate of aqueous humor formation and, subsequently, reduce the intraocular pressure. These compounds also have found some limited use in the treatment of absence seizures, to alkalinize the urine, to treat familial periodic paralysis, to reduce metabolic alkalosis, and prophylactically, to reduce acute mountain sickness. ACETAZOLAMIDE Synthesis Acetazolamide is 5-acetamido-1,3,4-thiadiazole-2-sulfonamide (9.7.5) The synthesis of acetazolamide is based on the production of 2-amino-5-mercapto-1,3, 4-thiadiazole (9.7.2), which is synthesized by the reaction of ammonium thiocyanate and hydrazine, forming hydrazino-N,N_-bis-(thiourea) (9.7.1), which cycles into thiazole (9.7.2) upon reaction with phosgene. Acylation of (9.7.2) with acetic anhydride gives 2-acetylamino-5-mercapto-1,3,4thiadiazol (9.7.3). The obtained product is chlorinated to give 2-acetylamino-5-mercapto-1,3,4thiadiazol-5-sulfonylchloride (9.7.4), which is transformed into acetazolamide upon reaction with ammonia (9.7.5) Structure activity relationship •Sulfamoyl group is essential for the carbonic anhydrase inhibitory activity in vitro and diueresis in Vivo •Substitution at sulfamoyl nitrogen leads to loss in activity Benzothiadiazine or Thiazide Diuretics Further study of the benzene disulfonamide derivatives was undertaken to find more efficacious carbonic anhydrase inhibitors. These studies provided some compounds with a high degree of diuretic activity. Chloro and amino substitution gave compounds with increased activity, but these compounds were weak carbonic anhydrase inhibitors. When the amino group was acylated, an unexpected ring closure took place. These compounds possessed a diuretic activity independent of the carbonic anhydrase inhibitory activity, and a new series of diuretics called the benzothiadiazines was discovered. Mechanism of Action The mechanism of action of the benzothiadiazine diuretics is primarily related to their ability to inhibit the Na+/Cl - symporter located in the distal convoluted tubule. These diuretics are actively secreted in the proximal tubule and are carried to the loop of Henle and to the distal tubule. The major site of action of these compounds is in the distal tubule, where these drugs compete for the chloride binding site of the Na+/Cl - symporter and inhibit the reabsorption of sodium and chloride ions. For this reason, they are referred to as saluretics. They also inhibit the reabsorption of potassium and bicarbonate ions, but to a lesser degree. Structure–Activity Relationship The thiazide diuretics are weakly acidic with a benzothiadiazine 1,1-dioxide nucleus. Chlorothiazide is the simplest member of this series, having a pKa of 6.7 and 9.5. The hydrogen atom at the 2-N is the most acidic because of the electron-withdrawing effects of the neighboring sulfone group. The sulfonamide group that is substituted at C-7 provides an additional point of acidity in the molecule but is less acidic than the 2-N proton. These acidic protons make possible the formation of a water -soluble sodium salt that can be used for intravenous administration of the diuretics. An electron-withdrawing group is necessary at posit ion 6 for diuretic activity. Little diuretic activity is seen with a hydrogen atom at position 6, whereas compounds with a chloro or trifluoromethyl substitution are highly active. The trifluoromethyl -substituted diuretics are more lipid-soluble and have a longer duration of action than their chloro-substituted analogues. When electron-releasing groups, such as methyl or methoxyl , are placed at position 6, the diuretic activity is markedly reduced. Replacement or removal of the sulfonamide group at position 7 yields compounds with little or no diuretic activity. Saturation of the double bond to give a 3,4-dihydro derivative produces a diuretic that is 10- fold more active than the unsaturated derivative. Substitution with a lipophilic group at position 3 gives a marked increase in the diuretic potency. Haloalkyl , aralkyl , or thioether substitution increases the lipid solubility of the molecule and yields compounds with a longer duration of action. Alkyl substitution on the 2-N position also decreases the polarity and increases the duration of diuretic action. Although these compounds do have carbonic anhydrase activity, there is no correlation of this activity with their saluretic activity (excretion of sodium and chloride ions) . Adverse Effects Thiazide diuretics may induce a number of adverse effects, including hypersensitivity reactions, gastric irritation, nausea, and electrolyte imbalances, such as hyponatremia, hypokalemia, hypomagnesemia, hypochloremic alkalosis, hypercalcemia, and hyperuricemia. Individuals who exhibit hypersensitivity reactions to one thiazide are likely to have a hypersensitivity reaction to other thiazides and sulfamoyl-containing diuretics (e.g., thiazide-like and some high-ceiling diuretics). Potassium and magnesium supplements may be administered to treat hypokalemia or hypomagnesemia, but their use is not always indicated. Combination preparation of hydrochlorothiazide or a potassium-sparing diuretic are available (e.g., Diazide and Moduretic). Long- term use of thiazide diuretics also may result in decreased glucose tolerance and increased blood lipid (low-density lipoprotein cholesterol , total cholesterol, and total triglyceride) content. High-Ceiling or Loop Diuretics Mechanism of Action This class of drugs is characterized more by its pharmacological similarities than by its chemical similarities. These diuretics produce a peak diuresis much greater than that observed with the other commonly used diuretics, hence the name high-ceiling diuretics. Their main site of action is believed to be on the thick ascending limb of the loop of Henle, where they inhibit the luminal Na+/K+/2Cl - symporter . These diuretics are commonly referred to as loop diuretics. Additional effects on the proximal and distal tubules also are possible. High-ceiling diuretics are characterized by a quick onset and short duration of activity. Their diuretic effect appears in approximately 30 minutes and lasts for approximately 6 hours. Structure–Activi ty Relationships Furosemide is an example of a high-ceiling diuretic and may be regarded as a derivative of anthranilic acid or o-aminobenzoic acid. Research on 5-sulfamoylanthranilic acids at the Hoechst Laboratories in Germany showed them to be effective diuretics. The most active of a series of variously substituted derivatives was furosemide. The chlorine and sulfonamide substitutions are features also seen in previously discussed diuretics. Because the molecule possesses a free carboxyl group, furosemide is a stronger acid than the thiazide diuretics (pKa = 3.9) . This drug is excreted primarily unchanged. A small amount of metabolism, however, can take place on the furan ring, which is substituted on the aromatic amino group Therapeutic Applications Furosemide has a saluretic effect 8- to 10-fold that of the thiazide diuretics; however, it has a shorter duration of action (~6–8 hours). Furosemide causes a marked excretion of sodium, chloride, potassium, calcium, magnesium, and bicarbonate ions, with as much as 25% of the filtered load of sodium excreted in response to initial treatment. It is effective for the treatment of edemas connected with cardiac, hepatic, and renal sites. Because it lowers the blood pressure similar to the thiazide derivatives, one of its uses is in the treatment of hypertension. Furosemide is orally effective but may be used parenterally when a more prompt diuretic effect is desired, such as in the treatment of acute pulmonary edema. The dosage of furosemide, 20– 80 mg/day, may be given in divided doses because of the short duration of action of the drug and carefully increased up to a maximum of 600 mg/day. Adverse Effects Clinical toxicity of furosemide and other loop diuretics primarily involves abnormalities of fluid and electrolyte balance. As with the thiazide diuretics, hypokalemia is an important adverse effect that can be prevented or treated with potassium supplements or coadministration of potassium-sparing diuretics. Synthesis Furosemide, 4-chloro-N-furfuryl-5-sulfamoylanthranylic acid (21.4.11), is synthesized in a relatively simple manner from 2,4-dichlorobenzoic acid, which is converted into 5-aminosulfonyl-4,6-dichlorobenzoic acid (21.4.10) during subsequent reaction with chlorosulfonic acid and ammonia. Reacting this with furfurylamine gives furosemide Potassium-Sparing Diuretics (Mineralocorticoid Receptor Antagonists)—Antihormone Diuretics Mechanism of Action The adrenal cortex secretes a potent mineralocorticoid called aldosterone, which promotes salt and water retention and potassium and hydrogen ion excretion. Other mineralocorticoids have an effect on the electrolytic balance of the body, but aldosterone is the most potent. Its ability to cause increased reabsorption of sodium and chloride ion and increased potassium ion excretion is approximately 3,000- fold that of hydrocortisone. A substance that antagonizes the effects of aldosterone could conceivably be a good diuretic drug. Spironolactone is such an antagonist. Metabolism On oral administration, approximately 90% of the dose of spironolactone is absorbed and is significantly metabolized during its first passage through the liver to its major active metabolite, canrenone, which is interconvertible with its canrenoate anion. Canrenone is an antagonist to aldosterone. Specific Drugs Spironolactone Spironolactone is a competitive antagonist to the mineralocorticoids, such as aldosterone. The mineralocorticoid receptor is an intracellular protein that can bind aldosterone. Spironolactone binds to the receptor and competitively inhibits aldosterone binding to the receptor. The inability of aldosterone to bind to its receptor prevents reabsorption of sodium and chloride ions and the associated water. The most important site of these receptors is in the late distal convoluted tubule and collecting system (collecting duct). The canrenoate anion is not active per se but acts as an aldosterone antagonist because of its conversion to canrenone, which exists in the lactone form. Canrenone has been suggested to be the active form of spironolactone as an aldosterone antagonist. The formation of canrenone, however, cannot fully account for the total activity of spironolactone. Both canrenone and potassium canrenoate are used as diuretics in other countries, but they are not yet available in the United States. Therapeutic Applicat ions Spironolactone is useful in treating edema resulting from primary hyperaldosteronism and refractory edema associated with secondary hyperaldosteronism. Spironolactone is considered to be the drug of choice for treating edema resulting from cirrhosis of the liver . The dose of spironolactone is 100 mg/day given in single or divided doses. Another use of spironolactone is coadministration with a potassium-depleting diuretic (e.g., a thiazide or loop diuretic) to prevent or treat diuretic-induced hypokalemia. Spironolactone can be administered in a fixed-dose combination with hydrochlorothiazide for this purpose, but optimal individualization of the dose of each drug is recommended. Adverse Effects The primary concern with the use of spironolactone is the development of hyperkalemia, which can be fatal. Spironolactone may cause hypersensitivity reactions, gastrointestinal disturbances, peptic ulcer, gynecomastia, decreased libido, and impotence. It also has been implicated in tumor production during chronic toxicity studies in rats, but human risk has not been documented.