* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Lab activity 8 Proteins 2 Alaa S Baraka Islamic university of Gaza
List of types of proteins wikipedia , lookup
Ribosomally synthesized and post-translationally modified peptides wikipedia , lookup
Cell-penetrating peptide wikipedia , lookup
Peptide synthesis wikipedia , lookup
Protein (nutrient) wikipedia , lookup
Western blot wikipedia , lookup
Metalloprotein wikipedia , lookup
Protein adsorption wikipedia , lookup
Genetic code wikipedia , lookup
Bottromycin wikipedia , lookup
Protein structure prediction wikipedia , lookup
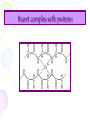
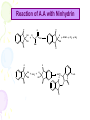
Lab activity 8 Proteins 2 Alaa S Baraka Islamic university of Gaza March2013 Color test of amino acids and proteins • A.A can be characterized qualitatively by using several dyes that will react with certain groups of the A.A. Lab activity • Sulfer test • Sakaguchi test • Ninhydrin test • Biuret test Sulfur test (Test for (-SH) group) (To detect amino acid which containe sulfur group) Principle • Proteins containing sulfur (cysteine and cystine and Methionine) give a black deposit of lead sulfide (PbS) when heated with lead acetate in alkaline medium. • Sulfur-containing protein ----> NaOH----> S2- ----Pb2+----> PbS Procedure 1. Add 1 ml of protein solution in a test tube, add 2 ml of 10% sodium hydroxide solution and 5 drops of lead acetate. 2. labble the tubes and shake them. then heat in a boiling water bath for 5 minutes. 3. Cool and record the results. Result: • A black deposit is formed with albumin • while a slight black turbidity is obtained with casein due to its lower content of sulfur. • Gelatin gives negative result. Sakaguchi Test for Arginine (detection of a specific type of protein with the amino acid containing the guanidinium group ) guanidinium group Principle: • α- naphthol and sodium hypobromite/chlorite react with guanidine group to form red orange complexes. • NaOBr+ >>>>>>>>>>>> Quinonَ Quinon + guanidine >>>>>>>>>>> Red complex Procedure • Add 1 ml of 3 N NaOH solution to 1 ml of the protein solution, followed by addition of 0.5 ml of 0.1 % αnaphthol solution, and a few drops of 2 % hypobromite solution (NaOBr). (For Biuret detecting peptide bonds ) Principle • The copper atoms of Biuret solution (CuSO4 ) in a basic environment will react with peptide bonds (-CO ---NH) to form a chelate of a deep violet color, indicating the presence of proteins. • A light pink color indicates the presence of peptides.. Biuret complex with proteins Procedure 1. To 1 ml of a solution containing protein add 4 ml of a biuret reagent. 2. Mix well, then let to stand at RT for about 30 min. Ninhydrin Test (general test for compounds with free a – amino groups) • For amino acids containing a free NH2 & free COOH. • Reaction with ninhydrin to produce a colored product. 1.When NH2 is attached to α-C on the amino acid’s carbon chain, the amino group’s N is part of a blue-purple product. 2.Amino acids that have N-H (a secondary amino group (e.g. proline) also react with ninhydrin, but they yield a yellow product. Reaction of A.A with Ninhydrin O O O OH OH + R H + OH RCHO + CO 2 + NH3 OH NH2 O O O O OH OH O + 2 NH3 + H + NH4 O O HO O + - N O O H2O Procedure.. 1. 2. 3. 4. Label 6 cleaned, drained test tubes with the names of the following solutions: 2 % glycine, 1 % tyrosine, 2 % proline, 2 % casein, 2 % gelatin, 2 % albumin. Add 15 drops of each solution in the corresponding test tube. To each of the test tubes add 5 drops of 0.5 % ninhydrin reagent solution. Place the test tubes into the boiling-water bath for 5 minutes. Remove the test tubes from the water bath and place then in a test tube rack. Record your observations! 17