* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Non-cardiac Surgery in the Adult Congenital Heart Patient
Survey
Document related concepts
Cardiac contractility modulation wikipedia , lookup
Electrocardiography wikipedia , lookup
Heart failure wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Coronary artery disease wikipedia , lookup
Cardiothoracic surgery wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Myocardial infarction wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Cardiac surgery wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Atrial septal defect wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
Transcript
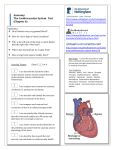
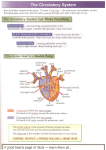
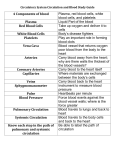
SCA 2013 PBLD 18: Non-‐ Cardiac surgery in the adult congenital heart patient Candice R. Montzingo, MD, FASE James A. DiNardo, MD, FAAP Associate Professor Chief, Division of Cardiac Anesthesia Dir of Education Cardiothoracic Division Children’s Hospital Boston Department of Anesthesiology Professor of Anesthesia University of Utah Harvard Medical School Salt Lake City, Utah Boston, MA Objectives: At the conclusion of this PBLD the participants will be able to: 1. Identify the difficulties of providing anesthetic care to an adult patient with congenital heart disease who is transitioning from a pediatric institution to an adult service. 2. Discuss an appropriate preoperative evaluation plan for an adult patient with Fontan physiology, presenting for a laparoscopic versus open tubal ligation. 3. Describe anesthetic concerns related to an adult with Fontan physiology and discuss a perioperative anesthetic plan. Case Presentation: A 22-‐year-‐old female presents to the Pre-‐surgical Anesthesia Evaluation Clinic 4 hours prior to a scheduled tubal ligation. Her surgeon has asked that she be evaluated to determine if she is a candidate for laparoscopic tubal ligation or if the procedure will have to be open. Upon questioning, she reports being born with hypoplastic left heart and that she “had several surgeries as a baby to correct it”. Since childhood, her surgical history is significant for pacemaker placement in 1992 with lead exchange in 2012. She wears 4 liters of oxygen by nasal cannula continuously and maintains saturations in the 85-‐89% range. On room air, she desaturates to 57%. The electronic medical record for this patient is blank, as she has received all previous hospital care at the local Children’s Hospital. Current medications include: Ambien, Celexa, Calcium and vit D, magnesium, vistaril, lactulose, magnesium oxide, spironolactone, tramadol, ursodiol and carvedilol. Family hx: she is adopted. Social history is non-‐contributory. 1. What are the next steps? What specific information needs to be obtained from the children’s hospital? 2. Describe hypoplastic left heart syndrome and the usual surgical correction technique. You request records from Children’s Hospital and are sent the most recent cardiology clinic note and the following image from the most recent catheterization report, done 6 months prior. The cardiology clinic note from 2 months ago confirms that she was born with hypoplastic left heart, dTGA, pulmonary atresia, discontinuous pulmonary arteries and bilateral SVC. She underwent staged palliation with a lateral tunnel Fontan and in 1993 her Fontan fenestration was occluded with a transcatheter device however, a small residual right to left shunt from the Fontan baffle to the right atrium persists. Other remarkable issues from the progress note include: Reduced RV function Liver enteropathy, liver cirrhosis with puritis and esophageal varices AV nodal reentrant tachycardia, s/p radiofrequency ablation Sinus node dysfunction, s/p AAIR pacemaker Chronic cyanosis Thrombocytopenia HPV Hypomagnesemia Chronic pain Upon further questioning, the patient reports sleeping for “a good part of the day” and the rest of her time is spent on the internet. She gets shortness of breath with most activity. She denies paroxysmal nocturnal dyspnea and orthopnea. She sleeps on 2 pillows. Her weight is stable and she does not have peripheral edema. She denies feeling any tachycardia or palpitations. She also denies syncope and lightheadedness. Her current vital signs are as follows: BP 88/60, HR 95, Temp 36.9 °C, RR 16, SpO2 85%, Height 1.626m, Weight 55.0 Kg. On physical exam, breath sounds are clear to auscultation and her heart is regular in rate and rhythm with a slight systolic murmur at the sternum void of diastolic murmurs. Capillary refill is brisk and her extremities are pink and warm to the touch. 1. What are anesthetic concerns for a patient with Fontan physiology? 2. Do you proceed with the planned tubal ligation in 1 hour, or do you wish to have further testing performed? 3. What anesthesiologist is going to be assigned to this case? 4. The operating room staff wants to know if they should get the open set or laparoscopic set of instruments on the case cart. Which do you suggest? 5. What is the plan for induction and intraoperative anesthetic plan for a patient with Fontan physiology? 6. Are there any postoperative considerations for this patient? Discussion: Hypoplastic left heart syndrome is a cyanotic congenital heart condition that occurs when left sided heart structures do not develop completely. The incidence of HLHS in the United States is 0.16-‐0.36 per 1000 live births and accounts for 2-‐3% of all congenital heart defects. 1 Although a constellation of defects can be seen, most commonly there is a hypoplastic ascending aorta and aortic arch, hypoplastic left ventricle, a large patent ductus arteriosus and an atrial septal defect. At tertiary centers, HLHS is diagnosed on prenatal ultrasound in 50-‐60 percent of cases allowing for counseling and preparation for delivery. Although studies have failed to demonstrate a difference in mortality between neonates diagnosed in the prenatal and postnatal period when born in a tertiary care facility.3 Hypoplastic left heart syndrome is a uniformly fatal condition within the first weeks of life if no intervention is made. Death occurs if a mixing lesion between the right side and the left side of the heart is absent. Typically patients with Hypoplastic Left Heart Syndrome (HLHS) are born with a patent foramen ovale (PFO) or non-‐restrictive atrial septal defect to allow mixing at the atrial level, in addition to a patent ductus arteriosus between the aorta and the pulmonary artery. The ductus arteriosus must remain open for blood to get to the systemic circulation from the right ventricle so infants are often started on prostaglandin E1. In addition, the PFO must be large enough for blood to re-‐enter the right side of the heart from the lungs. Approximately 10 percent of infants with HLHS have restrictive atrial septal defects that inhibit appropriate blood flow from the lungs to the right ventricle leading to pulmonary congestion and inadequate mixing of deoxygenated and oxygenated blood in the atria. 2 Special consideration must be given to the relationship between the systemic vascular resistance and the pulmonic vascular resistance as both the systemic and pulmonary vascular beds are receiving blood from the pulmonary artery. Following birth, the decrease in pulmonary vascular resistance results in a higher percentage of blood going to the lungs decreasing systemic blood flow. In addition, supplemental oxygen is often avoided as it could result in more blood flow to the lungs and not to the systemic circulation. Coronary and cerebral blood flow also comes from flow through the PDA and then traverses in a retrograde manner through the aortic arch and ascending aorta. Therefore, when systemic blood flow decreases, flow to the heart and brain is diminished as well. Babies born with HLHS must undergo staged reconstruction to palliate their condition. Three stage one procedures exist including the Norwood procedure, the Sano procedure and the hybrid procedure. Typically the Norwood Stage 1 is performed in the first few days of life to connect the single ventricle to the systemic circulation and to divert the pulmonary blood flow to the lungs via a conduit. First described in 1981, it involves constructing a neo aorta from the root of the pulmonary artery, ascending aorta and homograft tissue. This allows blood to be pumped from the single ventricle to the systemic circulation through the native pulmonic valve. Pulmonary blood flow occurs through a modified Blalock-‐Taussig shunt connecting the subclavian artery or innominate artery to the proximal right pulmonary artery. Finally, resection of the intra-‐atrial septum is frequently performed to ensure unobstructed flow from the pulmonary veins into the systemic right ventricle. Although the Norwood procedure is among the highest risk category if the Risk Adjustment in Congenital Heart Surgery scoring system, improved survival rates in the last decade approach 90 percent. 4 The SANO procedure is an alternative to the classic Norwood stage 1 procedure developed to decrease the diversion of blood from the systemic circulation seen in the Blalock Taussig shunt. In the SANO procedure, a conduit is created between the right ventricle and the pulmonary artery. In early studies, this procedure had better transplantation free survival rates at one year than the Norwood procedure; however, this group also had more complications die to substantially diminished pulmonary artery blood flow and after 12 months, the survival rates showed no significant advantage. Most recently described is the hybrid procedure. This procedure attempts to decrease the complications, which may be seen in the postoperative period following a Norwood or SANO procedure related to prolonged cardiopulmonary bypass and circulatory arrest. Both situations complicate sustaining a balance between the pulmonary and systemic circulations. The hybrid procedure involves using transcatheter techniques to place a stent into the ductus arteriosus to achieve systemic circulation; using bands on the right and left pulmonary arteries to restrict pulmonary blood flow; and performing a balloon atrial septostomy to alleviate restricted blood flow from the pulmonary veins to the systemic right ventricle. The second stage of reconstruction for HLHS is the hemi Fontan, or bidirectional Glenn. This typically occurs within the first 3-‐6 months of age. This procedure eliminates the shunt to the lungs by directly connecting the superior vena cava to the right pulmonary artery; venous blood passively flows from the superior vena cava into the pulmonary circulation. This alleviates the need for the right ventricle to pump blood to the lungs, offloading volume from the right ventricle. The patient remains cyanotic however, because the venous return from the inferior vena cava continues to enter the right ventricle and hence the systemic circulation. Stage III is the Fontan procedure and is often performed at 18 months -‐4 years of age. In an extracardiac Fontan procedure, the inferior vena cava is divided from the heart and anastomosed using a conduit to the pulmonary artery. Then a small fenestration is made between the right atrium and the conduit to decrease the likelihood of developing a pleural effusion by serving as a “pop-‐off” when the pulmonary vascular resistance becomes significantly elevated. This ensures the cardiac output is spared by deoxygenated blood through this right to left shunt. A lateral tunnel Fontan may also be performed in which a baffle is placed inside the atrium to direct blood from the inferior vena cava to the lungs. Many studies have investigated the risks and benefits of the fenestration in the Fontan circulation and at 20 year follow up, adult patients with patent fenestrations appear to have improved cardiac output and a lower incidence of atrial tachyarrhythmias.5 The extracardiac conduit was initially performed to minimize risks of atrial arrhythmias by decreasing suture lines in the atrium. However, as time has passed, it appears that patients with extracardiac conduits continue to get atrial arrhythmias that are more challenging to manage without the ability to use transvenous atrial pacing for sinus node dysfunction and transcatheter access for refractory arrhythmias.6 Cardiac transplantation is often not performed in these infants due to the lack of newborn organs, the incidence of rejection, the relatively short expected life span of the transplanted heart and the improved outcomes of staged reconstruction. Once a patient has Fontan physiology, the anesthesiologist must consider affects of the anesthetic agents, type of surgery and pulmonary mechanics when an anesthetic plan is developed. A low pulmonary vascular resistance remains critical as blood flow to the lungs remains passive. In addition, over time the right ventricle becomes hypertrophied and exhibits signs of failure. This is because the anatomic right ventricle is not equipped to pump against the much higher systemic vascular resistance over the lifetime of the patient. Cardiac output is determined by the transpulmonary gradient in Fontan physiology. This means that the gradient between central venous pressure and the end diastolic pressure of the systemic ventricle is the primary force promoting forward blood flow and cardiac output. Determinants of adequate blood flow in a patient with Fontan physiology are systemic venous pressure and volume, pulmonary vascular anatomy and pulmonary vascular resistance, atrioventricular valve function, cardiac rhythm and systemic ventricular function (systolic and diastolic). Compromise of any of these factors will also compromise cardiac output. Ventricular preload is of particular importance to maintain cardiac output. Patients with Fontan physiology often have baseline venoconstriction to maximally augment preload and therefore anesthetics that cause venodilitation can be detrimental to cardiac output and lead to cardiac instability. The physical exam of a patient with well functioning Fontan physiology would reveal acyanotic, warm, well perfused skin; palpable peripheral arterial pulses; no murmur on cardiac auscultation; and an arterial oxygen saturation of 90-‐95%. Complications of Fontan physiology include fatal arrhythmias (commonly intra atrial reentrant tachycardia), heart failure, thrombolic events, and sepsis. Patients with a failing Fontan often show signs of fatigue, decreased activity level, palpitations, syncopal episodes, weight gain, dyspnea and oxygen saturations less than 90%. Hypoxemia in patients with Fontan physiology commonly result from persistent right to left shunting and recurrent pleural effusions. Late disease processes in Fontan patients include plastic bronchitis and protein losing enteropathy. Protein losing enteropathy is a cardinal sign of a failing Fontan and is associated with a 30% mortality at 2 years. Systemic to pulmonary artery collaterals and pulmonary arteriovenous malformations often develop in cyanotic Fontan patients. These can lead to pulmonary hypertension, intrapulmonary shunting and severe systemic hypoxemia. Attempts should be made to coil or device occlude these vascular anomalies. The following figure is a schematic representation of a normal, 2-‐ventricle series circulation; single ventricle parallel circulation; and a Fontan series circulation. The energy required to overcome systemic vascular resistance in a normal circulation is supplied by the left ventricle. In the single ventricle circulation, the systemic ventricle is presented with a large volume overload and supplies the energy to overcome both the systemic and pulmonary circulations arranged in parallel. Parallel arrangement of 2 resistances requires one-‐fourth the energy necessary to provide the same flow as in a circuit where the 2 resistances are arranged in series. In the Fontan circulation, the systemic ventricle faces both the systemic and pulmonary resistances arranged in series with the systemic venous circulation and the total cavopulmonary connection (TCPC) pathway interposed between the two.8 Schematic representations of normal 2-‐ventricle circulation (A), single-‐ventricle physiology (B), and Fontan physiology (C)a Abbreviations: SV, single ventricle; LV, left ventricle; RV, right ventricle; RS, systemic vascular resistance; RP, pulmonary vascular resistance; Ao, aorta; LA, left atrium; RA, right atrium; PA, pulmonary artery. 8 Three resistance beds (systemic vascular bed, the TCPC pathway, and the pulmonary vascular bed) are arranged in series in Fontan circulation demonstrating why afterload is elevated. Therefore, even Fontan patients with normal ventricular function have contractility afterload mismatch even at rest.8 References: 1. Karamlou T, Diggs BS, Ungerleider RM, Welke KF. Evolution of treatment options and outcomes for hypoplastic left heart syndrome over an 18-‐year period. J Thorac Cardiovasc Surg. 2010; 139 (1):119. 2. Vlahos AP, Lock JE, McElhinney DB, van der Velde ME. Hypoplastic left heart syndrome with intact or highly restrictive atrial septum: outcome after neonatal transcatheter atrial septostomy. Circulation. 2004;109(19):2326. 3. Mahle WT, Clancy RR, McGaurn SP, Goin JE, Clark BJ. Impact of prenatal diagnosis on survival and early neurologic morbidity in neonates with hypoplastic left heart syndrome. Pediatrics.2001;107(6):1277. 4. Jenkins KJ. Risk adjustment for congenital heart surgery: the RACHS-‐1 method. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2004;7:180. 5. Eagle SS, Daves SM. The adult with Fontan physiology: Systematic approach to perioperative management for non cardiac surgery. JCVA.2011;25(2):320-‐ 334. 6. Khairy P, Poirier N. Is the extracardiac conduit the preferred Fontan approach for patients with univentricular hearts?: The extracardiac conduit is not the preferred Fontan approach for patients with univentricular hearts. Circulation.2012;126:2516-‐2525. 7. McClain CD, McGowan FX, Kovatsis PG. Laparoscopic surgery in a patient with Fontan physiology. Anesth Analg 2006;103:856-‐8. 8. DiNardo JA. Heart failure associated with adult congenital heart disease. Semin Cardiothorac Vasc Anesth. December 2012. 9. Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 guidelines for the management of adults with congeni-‐ tal heart disease: a report of the American College of Cardi-‐ ology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease). Developed in Collaboration With the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Soci-‐ ety of Thoracic Surgeons. J Am Coll Cardiol. 2008;52: e1-‐e121.