* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Optical mapping of activation patterns in an animal - AJP
Quantium Medical Cardiac Output wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Heart failure wikipedia , lookup
Rheumatic fever wikipedia , lookup
Myocardial infarction wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Cardiac surgery wikipedia , lookup
Jatene procedure wikipedia , lookup
Congenital heart defect wikipedia , lookup
Atrial septal defect wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
Electrocardiography wikipedia , lookup
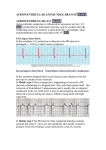
Am J Physiol Heart Circ Physiol 280: H1889–H1895, 2001. Optical mapping of activation patterns in an animal model of congenital heart block MARK RESTIVO,1,2 DMITRY O. KOZHEVNIKOV,1 AND MOHAMED BOUTJDIR1,2 Molecular and Cellular Cardiology Program, Veterans Affairs New York Harbor Health Care System, and 2State University of New York Health Science Center, Brooklyn, New York 11209 1 Received 18 May 2000; accepted in final form 8 November 2000 (8) and McCue et al. (18) noted more than 20 years ago that women who gave birth to children with congenital heart block (CHB) often had autoimmune diseases. It is now well established that CHB detected before or at birth, in the absence of structural cardiac abnormalities, is strongly associated with maternal antibodies to SSA/Ro and/or SSB/La ribonucleoproteins (6). Though more prevalent in the presence of autoimmune disorders, CHB in offspring is independent of whether the mother has systemic lupus erythematosus, Sjögren’s syndrome, or is totally asymptomatic (6, 26). Damage to the cardiac conduction system occurs in an otherwise normal fetus and is presumed to arise from transplacental passage of these maternal autoantibodies (immunoglobulin; IgG) (25). Other neonatal abnormalities affecting the skin, liver, and blood cells have also been reported (26) and are associated with these maternal IgG. Although varying degrees of conduction block can occur, third-degree atrioventricular (AV) block, which is irreversible, carries substantial morbidity and mortality that approaches 30%. More than 60% of the children affected by AV block require lifelong pacemakers (26). Noncardiac manifestations are transient, resolving ⬃6 mo after birth, coincident with the disappearance of maternal IgG from infant circulation (7). The candidate antigens and their cognate antibodies have been extensively characterized at the molecular level. The 60-kDa SSA/Ro contains a putative zinc finger and an RNA-binding protein consensus motif (2), both of which could account for its direct interaction with small cytoplasmic hY-RNAs, a class of low-molecular-weight molecules of 83–112 bases, which are small uncapped cytoplasmic RNA (16). This protein may function as part of a novel quality control or discard pathway for 5S rRNA precursors in Xenopus oocytes (22). Many sera, which recognize the 60-kDa SSA/Ro protein, also react with another protein of 52 kDa (1). Anti-SSB/La antibodies recognize a 48-kDa polypeptide, which does not share antigenic determinants with either 52-kDa or 60-kDa SSA/Ro (9). SSB/La facilitates the maturation of RNA polymerase III transcripts, directly binds a spectrum of RNAs, and associates at least transiently with 60-kDa SSA/Ro (13). We recently (3, 4) provided evidence that IgG-enriched fractions and anti-52-kDa SSA/Ro antibodies affinity purified from sera of mothers whose children have CHB induce complete AV block in Langendorff perfused hearts and inhibit the L-type Ca2⫹ current (ICa,L). In addition, immunization of female BALB/C mice with recombinant ribonucleoproteins generated high-titer antibodies, which crossed the placenta during pregnancy and were associated with varying degrees of AV conduction abnormalities in the pups, including complete AV block (4, 17, 20). We unexpectedly found significant sinus bradycardia in mouse pups born to mothers injected with human maternal anti- Address for reprint requests and other correspondence: M. Restivo, Dept. of Veterans Affairs, New York Harbor Health Care System, Cardiology Division (111A), 800 Poly Pl., Brooklyn, NY 11209 (E-mail: [email protected]). The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked ‘‘advertisement’’ in accordance with 18 U.S.C. Section 1734 solely to indicate this fact. cardiac electrophysiology; autoantibodies; atrioventricular node CHAMEIDES ET AL. http://www.ajpheart.org H1889 Downloaded from http://ajpheart.physiology.org/ by 10.220.33.1 on May 6, 2017 Restivo, Mark, Dmitry O. Kozhevnikov, and Mohamed Boutjdir. Optical mapping of activation patterns in an animal model of congenital heart block. Am J Physiol Heart Circ Physiol 280: H1889–H1895, 2001.—Congenital heart block (CHB) is associated with high mortality and affects children of mothers with autoantibodies (IgG) to ribonucleoproteins SSB/La and SSA/Ro. IgG from mothers of children with CHB (positive IgG) was used to assess activation patterns in both the right atrium (RA) and right ventricle (RV) of Langendorff-perfused young rabbit hearts. Optical action potentials (AP) were obtained by using a 124-site photodiode array with 4-[-[2-(di-n-butylamino)-6-naphthyl]vinyl]pyridinium. Optical APs were recorded to simultaneously image activation patterns from the RA and RV. Perfusion of positive IgG (800–1,200 g/ml) resulted in sinus bradycardia and varying degrees of heart block. Activation maps revealed marked conduction delay at the sinoatrial junction but only minor changes in overall atrial and ventricular activation patterns. No conduction disturbances were seen in the presence of IgG from mothers with healthy children. In conclusion, besides atrioventricular (AV) block, positive IgG induces sinus bradycardia. These results establish that the sequelae of CHB are associated with impaired intrasinus and/or sinoatrial conduction. The findings raise the possibility that sinus bradycardia in the developing heart may indicate the potential for AV conduction disturbances. H1890 OPTICAL MAPPING AND CONDUCTION ABNORMALITIES METHODS Surgical procedure and experimental setup. The rabbit model was chosen for several reasons. First, the dimensions of the heart are well suited for the spatial resolution of the optical system and permit imaging of large planar surfaces of the heart and specific cardiac structures (15, 24). Second, the optical resolution of the system can be adjusted from 400 to Fig. 1. Experimental setup. A: view of right atrium and ventricle. B: superimposition of optical grid on heart. 1, left ventricle; 2, right ventricle (RV); 3, left atrium; 4, right atrium (RA); 5, superior vena cava (SVC); 6, inferior vena cava (IVC); and 7, aorta. 1,800 M/pixel, depending on the dimension of the region of interest, and electrophysiological observations can be correlated with high resolution with the anatomic features of the heart (10). Third, the electrophysiological properties of the ionic currents that constitute the rabbit action potential (AP) have been well characterized and may better represent the human heart rather than smaller animals (21). Young New Zealand White rabbits of either sex, weighing 1.5–2 kg, were anesthetized by intravenous injection of fentanyl citrate (100 g/kg ⫹ 15 g 䡠 kg⫺1 䡠 h⫺1) with heparin sodium (1,000 U/kg). A tracheotomy was performed and the animal intubated with an endotracheal tube. The rabbit was ventilated with room air via a positive pressure ventilator (MD Industries; Mobile, AL). A midline thoracotomy was performed and the heart was exposed in a pericardial cradle. The caval veins were isolated and loose ligatures were placed around the inferior and superior vena cavae. The heart was rapidly excised and placed in cold oxygenated Tyrode solution containing 1,500 U/l heparin sodium. The heart was then cannulated and retrogradely perfused in a modified Langendorff setup and optical perfusion chamber. The animal procedures conformed to the principles embodied in the Declaration of Helsinki. All of the experimental protocols were approved by the Animal Studies Subcommittee of the Research and Development Department of the Department of Veterans Affairs, New York Harbor Healthcare System, and all procedures related to animal use comply with the Guiding Principles for Research Involving Animals and Human Beings. The perfused hearts were positioned in a fluid-filled Plexiglas chamber, as previously described (24). Placing the heart against an optically clear glass surface minimizes spatial aberration and provides for the highest fidelity signals while minimizing motion artifacts. A plastic cannula was inserted through the right atrium from the inferior vena cava to the superior vena cava (see Fig. 1A). Ligatures at the caval veins and a ligature at the tip of the right atrial appendage were affixed to three posts within the chamber. This provided for a smooth surface for optical imaging. Figure 1B shows the perfused heart setup. The heart was situated against the imaging surface by positioning a Lexan plunger posterior to Downloaded from http://ajpheart.physiology.org/ by 10.220.33.1 on May 6, 2017 bodies (17) or immunized with recombinant antigens (4, 20). These findings brought clinical attention not only to AV nodal disorders but also to sinoatrial (SA) nodal conduction abnormalities. In this regard, Brucato et al. (5) recently reported similar sinus bradycardia in infants born to mothers seropositive to antiSSA/Ro antibodies, suggesting that the spectrum of conduction abnormalities associated with maternal IgG extend beyond the AV node. The electrophysiological mechanisms by which maternal antibodies affect electrical propagation throughout the intact heart remain unclear. This may be due in part to the inability to map electrical activity patterns by using conventional microelectrode techniques in isolated multicellular preparations (3). Furthermore, whereas the primary electrophysiological consequence of maternal antibodies is AV block, little, if anything, is known about the effects of these maternal IgG on both atrial and ventricular activation and repolarization patterns. Sinus bradycardia might well be an early manifestation of CHB and may prove to be a critical clinical marker preceding the onset of advanced AV conduction disorders in autoimmune-associated CHB. Driven by these observations, we mapped cardiac activation patterns in Langendorff-perfused young rabbit hearts (atria and ventricles) exposed to maternal IgG. Optical mapping techniques, which reveal the twodimensional spread of activation, were used to study propagation abnormalities by using a 124-element photodiode array during sinus and paced rhythms. H1891 OPTICAL MAPPING AND CONDUCTION ABNORMALITIES immunoblot (3, 4, 17, 20). This will be referred to in the text as positive IgG. Control IgG (negative IgG) was purified from sera of healthy mothers (n ⫽ 3) with healthy children who tested negative for anti-SSA/Ro and anti-SSB/La antibodies by ELISA and immunoblot, as described above (3, 4, 17, 20). An IgG concentration of 800–1,200 g/ml was used in the experiments. The selected doses were based on our previous observations (3), in which a complete AV block was induced at 800 g/ml in the rat heart. Data analysis. All data are presented as means ⫾ SE. Statistical tests were performed by using Systat for Windows, version 8 (SPSS; Chicago, IL). AP characteristics were compared by Student’s t-test for paired and unpaired data where appropriate. Differences in activation time (total atrial/ventricular activation time) and cardiac intervals (P-R interval and R-R interval) were compared by ANOVA. Data before and after IgG application were compared by using ANOVA for repeated measures. If ANOVA failed to reach statistical significance, Bonferroni’s correction for paired data was also applied to test for effect. Optical APs were mapped by using a photodiode array (124 sites) and the fluorescent dye di-4-ANEPPS. The time of atrial or ventricular activation for each optical AP in the grid corresponded to the peak first temporal derivative of the upstroke (phase 0). The rate of change of fluorescence (dF/dt) was computed by using a four-point central difference formula and was applied to the normalized optical signal. Repolarization corresponded to 90% recovery of the normalized optical AP duration (APD90) (11). For this study, APD90 is defined as the difference between these intervals. Peak dF/dt and APD90 were computed by using a sampling interval of 0.64 ms. Isochronal activation maps were constructed with contours drawn at 1- to 3-ms intervals. Analysis was both descriptive and quantitative. RESULTS In the present study, 15 isolated hearts were used with 3 hearts used for sham study. Ten experimental hearts were exposed to 800 g/ml positive IgG, and in two experiments a dose of 1,200 g/ml positive IgG was used. Of the 10 hearts exposed to 800 g/ml positive IgG, 4 hearts were studied by using a high data acquisition rate (0.64 ms/sample) to compare dF/dt and APD90 before and after IgG. In the sham experiments, hearts were exposed to 1,200 g/ml negative IgG (i.e., control IgG obtained from healthy mothers with healthy children) and considered as controls for positive IgG. As shown in Table 1, there was no significant change in ECG parameters from control in these hearts. In 9 of 12 hearts exposed to positive IgG, complete heart block (3° AV block) was obtained. Only Table 1. Summary of electrocardiographic measurements in hearts with and without IgG n Interval, in ms Control IgG Positive IgG† 9 Negative IgG 3 R-R P-R R-R P-R 380 ⫾ 25 63 ⫾ 6 337 ⫾ 26 55 ⫾ 4 781 ⫾ 67* 121 ⫾ 9* 344 ⫾ 18 56 ⫾ 6 Values are means ⫾ SE; n, no. of hearts. All IgG measurements were made 15 min after start of perfusion. * P ⬍ 0.05 compared with control. † Three rabbits developed 3° atrioventricular block before electrocardiographic measurements could be performed. Downloaded from http://ajpheart.physiology.org/ by 10.220.33.1 on May 6, 2017 the imaged plane. The plunger also served to seal the chamber, and the exterior of the heart was bathed in the effused Tyrode solution. A probe was positioned within the chamber to monitor temperature. With this experimental setup, epicardial temperature gradients were ⬍0.2°C (15). Hearts were perfused with oxygenated (95% O2-5% CO2) modified Tyrode solution containing (in mM) 130 NaCl, 25 NaHCO3, 1.20 MgSO4, 4.75 KCl, 10 dextrose, and 1 CaCl2 (pH 7.4, 37°C). Perfusion pressure was maintained at 70–80 mmHg by adjusting the flow rate of a constant flow peristaltic pump. Hearts were stained with a voltage-sensitive dye, 4-[-[2-(di-n-butylamino)-6-naphthyl]vinyl]pyridinium (di-4ANEPPS, Molecular Probes; Eugene, OR) by injection of 150–200 l (from a 3 mM stock solution) into an air bubble trap (5-ml volume) immediately proximal to the cannulated heart. The dye was infused over a 5-min period. Data were acquired after a 15-min stabilization period. The surface of the heart was illuminated with the use of two halogen light sources. Light was passed through 520 ⫾ 20-nm interference filters, collimated, and focused on the heart surface with an incidence angle of ⬃45°. Fluoresced and scattered light was collected from the stained heart with the use of a high numerical aperture, epi-illumination lens (50 mm, 1:1.4 E series, Nikon; Garden City, NJ). Collected light was passed through a 645-nm cutoff filter (model RG645; Schott Glass) and focused onto a 12 ⫻ 12 photodiode array (Centronics; Newbury Park, CA). The physical dimensions of the photodiode array were 18 ⫻ l8 mm; the dimension was 1.4 ⫻ l.4 mm per diode with a 0.1-mm dead space between each diode. At ⫻1.5, the signal from each diode corresponded to an area of ⬃1 ⫻ 1 mm. The depth of field was ⬃200 m (15). Other details of the optic arrangement can be found elsewhere (15, 24). Amplified signals were multiplexed and analog to digital converted and transferred to a Pentium computer (Dimension XPS-T550, Dell Computers; Round Rock, TX). Data were filtered at a band-pass range of 0.05–1,000 Hz. Because optical signals cannot be monitored continuously and because the occurrence of spontaneous events cannot be predicted a priori, a circular memory buffer was used to acquire data with an adjustable pretrigger of up to 40 s. The digitized data were monitored and then transferred to the computer hard drive after each epoch. At the end of each experiment, data were archived on a recordable CD-ROM drive (model HP8100, Hewlett-Packard; Palo Alto, CA). Stimulation and electrocardiogram. Stimulation electrodes were placed at the right atrial appendage near the superior vena cava. The bipolar stimulation electrodes were composed of Teflon-coated silver wires (75-m diameter) with an exposed length of 500 m. The hooked ends were inserted into the myocardium with an interpolar distance of ⬍0.5 mm. Stimulation was applied from a constant current source at two times diastolic threshold. An electrocardiogram (ECG) was recorded from two electrodes located in positioning plungers at opposite sides of the heart. The ECG was filtered at a band pass of 0.05–250 Hz and monitored continuously on a digital oscilloscope (model 2522A, BK Precision; Chicago, IL). The same ECG was also recorded simultaneously with the optical signals. IgG purification. Purification of IgG was performed as previously described (3, 4, 17, 20). Briefly, immunoglobulin fractions containing IgG were purified from serum by protein A-sepharose columns and confirmed pure by electrophoresis. Throughout this study, we tested IgG from mothers (n ⫽ 3) whose children have CHB and contain antibodies against 48-kDa SSB/La, 52-kDa SSA/Ro, and 60-kDa SSA/Ro, as tested by enzyme-linked immunosorbent assay (ELISA) and H1892 OPTICAL MAPPING AND CONDUCTION ABNORMALITIES Table 2. dF/dt and APD90 values before and after positive autoantibodies Atrium Control IgG %Change Ventricle n APD90 dF/dt APD90 dF/dt 4 4 78 ⫾ 0.3 75 ⫾ 0.5* ⫺5 1.34 ⫾ 0.07 1.30 ⫾ 0.04† ⫺4.1 128 ⫾ 0.4 151 ⫾ 2.0* 15 0.94 ⫾ 0.03 0.93 ⫾ 0.07† ⫺0.7 Values are means ⫾ SE; n, no. of hearts. APD90, 90% recovery of normalized optical action potential duration; dF/dt, rate of change of fluorescence. All IgG measurements were made 15 min after start of perfusion. Student’s t-test for paired data. * P ⬍ 0.05 compared with control. † Not statistically significant. Fig. 2. Electrocardiogram (ECG) recordings from Langendorff perfused rabbit heart before and after positive immunoglobulin (IgG) (800 g/ml). A: control; B: slowing of sinus rate after positive IgG; C: second-degree heart block in the same heart with a 2:1 pattern, which later evolved into 4:1 AV block (D). After washout, sinus bradycardia persisted (E). Arrows, P waves. Downloaded from http://ajpheart.physiology.org/ by 10.220.33.1 on May 6, 2017 partial recovery was observed when the hearts were perfused with positive IgG-free modified Tyrode solution for up to 45 min. The summary of ECG measurements is shown in Table 1. Table 2 compares dF/dt and APD90 before and after positive IgG for four hearts. In these experiments, the same six sites were selected and the data were pooled (n ⫽ 24). The right atrial sites were chosen from the free right atrial wall, distant from the vicinity of the SA. There was a modest increase of 5% in APD90 in the right atrium and an increase of 15% in the imaged portion of the right ventricle. Both effects were statistically significant. On the other hand, there was no statistically significant effect of positive IgG on dF/dt in both the right atrium and ventricle. Figure 2 shows ECG recordings from a typical experiment that produced CHB in the presence of positive IgG (800 g/ml). The control ECG shows normal sinus rhythm with a P wave, followed by a QRS complex at a spontaneous heart rate of 160 beats/min (Fig. 2A). The P-R interval was 67 ms. Fifteen minutes after infusion of positive IgG, the heart rate slowed to 113 beats/min and the P-R interval increased to 180 ms (Fig. 2, trace B). Note that the QRS complex duration was essentially unaffected by positive IgG infusion. Fig. 2, traces C and D, shows 2° AV block developed 19 min after start of positive IgG infusion and 2° AV block with a 4:1 pattern at 24 min, respectively. Figure 2E shows partial recovery of heart block 45 min after perfusion with positive IgG-free Tyrode solution. However, sinus bradycardia and 1° AV block at a heart rate of 138 beats/ min were maintained. Figure 3 shows atrial and ventricular activation maps, along with selected optical APs during fixed atrial pacing (A1⫺A1 ⫽ 250 ms) in the presence of positive IgG (800 g/ml). Optical AP were mapped using a photodiode array (124 sites) and di-4-ANEPPS. Stimulation was applied in the sinus node region (see asterisk in Fig. 3). The map projections shown in the top panels are right-sided projections of the epicardial heart surface in control (Fig. 3, left) and in the presence of positive IgG (Fig. 3, right). A rough schematic of the right atrium is outlined in the lower portion of the maps with anatomic landmarks indicated. Optical AP were acquired to simultaneously record from the right atrium and ventricle. In the activation maps, each shaded region correspond to an isochronal area acti- vated each successive millisecond. The arrows indicate the direction of activation wave front propagation. In the control right atrial map, total activation time was 4 ms; total activation time for the imaged portion of the right ventricle was 5 ms. Complete heart block was obtained after positive IgG infusion (Fig. 3, right). In the right atrial activation map, there was considerable delay at the SA junction, as indicated by the crowded isochrones. Whereas right atrial activation time was 9 ms, most of the delay was at the SA junction. The majority of the remaining atrium was activated within 3 ms, as in control. The right ventricular activation time for the ventricular escape beat was 4 ms. The ECG and a selected atrial and ventricular optical action potential during control (left) and positive IgG infusion (right) are shown at the lower portion of Fig. 3. The role of atrial conduction disturbances during spontaneous sinus rhythm in control and after positive IgG perfusion (1,200 g/ml) is shown in Fig. 4. The topology of these maps was similar for all six hearts that developed 3° AV block. The control right atrial map (Fig. 4, left) shows that the right atrium was activated within 5 ms. The spontaneous atrial rate was 180 beats/min with 1:1 AV conduction. The atrial isochrones were evenly spaced, indicating that there were no conduction disturbances in the right atrium. The right ventricle also activated within 5 ms. The activation pattern was from apex to base, indicating normal sinus activation of the ventricles. Perfusion of positive IgG resulted in sinus bradycardia, followed by complete AV block corresponding to an atrial rate of 144 OPTICAL MAPPING AND CONDUCTION ABNORMALITIES H1893 beats/min. Examination of the atrial activation map (Fig. 4, right) shows that sinus bradycardia was associated with marked conduction delay at the SA junction. The activation isochrones were crowded in the SA region, similar to the paced example shown in the Fig. 3. Total right atrial activation time increased to 7 ms. The right ventricle activated within 6 ms and was similar in activation sequence and timing to the control map. In this and all other experiments, sinus bradycardia was associated with only minor changes in overall atrial and ventricular activation patterns. To understand the electrophysiological effects of positive IgG better, optical APs were obtained at a higher magnification in the region of the sinus node and SA junction. The results from a representative experiment, in which complete AV dissociation was obtained in the presence of positive IgG (800 g/ml), are shown in Fig. 5. Optical APs are from one column of the grid. Figure 5, traces A-D, shows that most of the conduction occurred in the sinus node to SA junction region. In this zone, there was a reduced upstroke in optical AP. Optical AP from the free right atrial wall before and after positive IgG were similar, indicating that positive IgG had little effect on the rest of the atrium. Although there was a modest increase (15%) in APD90, there was no statistically significant effect on dF/dt at this con- Fig. 4. Optical maps of atrial and ventricular activation along with selected optical AP during spontaneous sinus rhythm in control and after positive IgG (1,200 g/ ml) perfusion. Left: control RA and ventricular activation maps. No conduction disturbances were evident in the right atrium in control. RV had a normal sinus activation pattern. Right: positive IgG caused third-degree heart block with a ventricular escape rhythm. Sinus bradycardia was associated with marked conduction delay at the sinoatrial junction as indicated by the crowded isochrones in the sinoatrial region. Maps and abbreviations are the same as in Fig. 3. Downloaded from http://ajpheart.physiology.org/ by 10.220.33.1 on May 6, 2017 Fig. 3. Optical maps of atrial and ventricular activation along with selected optical action potentials (AP) during fixed atrial pacing at a rate of 240 beats/min. Stimulation (*) was applied in the sinus node region. Rough schematic of RA is outlined in the lower portion of the maps with anatomic landmarks indicated. Activation maps are right-sided projections of epicardium in control (left) and in the presence of 800 g/ml positive IgG (right). Each isochronal zone (shaded region) corresponds to an isochronal time of 1 ms. Color code key for atrial and ventricular isochrone intervals is shown in between the 2 maps. H1894 OPTICAL MAPPING AND CONDUCTION ABNORMALITIES centration of positive IgG, indicating that IgG had little effect on the conduction in the imaged portion of the right ventricle. DISCUSSION The primary finding of this study is that positive maternal IgG causes bradycardia in the isolated Langendorff, perfused young rabbit heart. Whereas complete heart block is a hallmark of this potentially fatal congenital disorder, bradycardia was a consistent finding in this study. Along with slowing of the intrinsic sinus rate, optical mapping revealed marked conduction delays for propagation of the sinus impulse to the atrium. Comparison of the activation maps and dF/dt measurements indicates that there was no effect on conduction in other parts of the right atrium and the mapped portion of right ventricle. The fact that conduction is affected at the SA junction is not unlike the documented concept that these IgG target specific cardiac conduction systems in the heart, such as the AV node. The reason that sinus node and SA dysfunction have been overlooked until recently may be related to the severity of the disease during high-level AV nodal block and the limited number of electrophysiological studies in this relatively rare congenital disorder. Downloaded from http://ajpheart.physiology.org/ by 10.220.33.1 on May 6, 2017 Fig. 5. Optical AP obtained at a magnification of 0.4 mm/pixel along one column of the grid in the presence of positive IgG (800 g/ml) going from A (sinus node region) to L (free RV wall). Whereas optical mapping of hearts with CHB need to be investigated in detail at many levels, our attention to atrial and SA conduction disorders in the present study was precipitated by the recent and somewhat unexpected findings from our laboratory, that sinus bradycardia was more common in animal models of CHB than previously realized (4, 17). These findings have been extended to the clinical settings, where sinus node dysfunction in infants of mothers with anti-Ro antibodies has been documented (5, 19). It is tempting to speculate that because of the significant mortality and morbidity associated with AV node dysfunction, the role of sinus node dysfunction may well have been overlooked for years. There are two implications to the findings of this study. First, sinus bradycardia may have long been an early electrophysiological consequence in the CHB syndrome. Our results, along with the observations of others (5, 19), hopefully will draw more attention of health care providers to the potential critical role of sinus bradycardia for the early diagnosis of CHB. Second, because it appears that maternal IgG target specific conduction systems in the heart, there is a continued need for basic scientific investigation into the histopathogical mechanism by which these antibodies interact with systems responsible for the initiation of the cardiac impulse in the SA node and its propagation to the atria and ventricles. Proposed mechanisms of tissue injury. Whereas the molecular and biochemical mechanisms of CHB have been explored to a certain extent (see Ref. 7 for review), the electrophysiological mechanisms of CHB are just emerging (3, 4, 12, 17, 20). Work from our laboratory was based on the initial observation of Garcia et al. (12), who showed that the use of a single-surface ECG that conduction disorders associated with neonatal lupus could be reproduced in an isolated adult rabbit heart. They further showed that anti-SSA/Ro positive sera induced a reduction in ICa,L. Subsequently, and unlike Garcia et al. (12), we used a large panel of sera only from mothers whose children have CHB and tested their electrophysiological effects on human fetal heart (4). We provided evidence that IgG-enriched fractions and anti-52-kDa SSA/Ro antibodies affinity purified from sera of mothers whose children have CHB induce complete AV block in the fetal heart perfused by the Langendorff technique and inhibit ICa,L at the whole cell and single channel level (3, 4). In addition, immunization of female BALB/C mice with recombinant proteins generated high-titer antibodies, which crossed the placenta during pregnancy and were associated with varying degrees of AV conduction abnormalities, including complete AV block, in the pups (4, 17, 20). Because conduction in the SA and AV node is essentially dependent on Ca electrogenesis, sinus bradycardia, and AV block would be expected to result from interventions that reduce Ca2⫹ currents. The possibility that these IgG interact with other currents in the SA or AV node cannot be ruled out. We propose that chronic exposure of Ca2⫹ channels to maternal antibodies during pregnancy could lead to Ca2⫹ chan- OPTICAL MAPPING AND CONDUCTION ABNORMALITIES The authors thank Joyce Ince in the Animal Care Facility for technical assistance. IgG was obtained from the Research Registry for Neonatal Lupus. This work was supported by National Heart, Lung, and Blood Institute Grant HL-55401 (to M. Boutjdir) and Veterans Administration Medical Research Funds as Merit Grant Awards (to M. Boutjdir and to M. Restivo). REFERENCES 1. Ben-Chetrit E, Chan EKL, Sullivan KF, and Tan EM. A 52-kD protein is a novel component of the SS-A/Ro antigenic particles. J Exp Med 167: 1560–1571, 1988. 2. Ben-Chetrit E, Gandy BJ, Tan EM, and Sullivan KF. Isolation and characterization of a cDNA clone encoding the 60-kD component of the human SS-A/Ro ribonucleoprotein auto antigen. J Clin Invest 83: 1284–1292, 1989. 3. Boutjdir M, Chen L, Zhang ZH, Tseng CE, El-Sherif N, and Buyon JP. Serum and immunoglobulin G from the mother of a child with congenital heart block induce conduction abnormalities and inhibit L-type calcium channels in a rat heart model. Pediatr Res 44: 11–19, 1998. 4. Boutjdir M, Chen L; Zhang ZH, Tseng CE, DiDonato F, Rashbaum W, Morris A, El-Sherif N, and Buyon JP. Arrhythmogenicity of IgG and anti-52-kD S. SA/Ro affinity-purified antibodies from mothers of children with congenital heart block. Circ Res 80: 354–362, 1997. 5. Brucato A, Cimaz R, Catelli L, and Meroni P. Anti-Ro associated sinus bradycardia in newborns. Circulation 102: E88– H89, 2000. 6. Buyon JP, Winchester RJ, Slade SG, Arnett F, Copel J, Freidman D, and Lockshin MD. Identification of mothers at risk for congenital heart block and other neonatal lupus syndromes in their children: comparison of ELISA and immunoblot to measure anti-SSA/Ro and anti-SSB/La antibodies. Arthritis Rheum 36: 1263–1273, 1993. 7. Buyon JP. Neonatal lupus syndromes. In: Systemic Lupus Erythematousus, edited by Lahita R. New York: Churchill Livingstone, 1999, p. 337–359. 8. Chameides L, Truex RC, Vetter V, Rashkind WJ, Galioto FM, and Noonan JA. Association of maternal systematic lupus erythematosus with congenital complete heart block. N Engl J Med 297: 1204–1207, 1977. 9. Chan EKL, Francoeur AM, and Tan EM. Epitopes, structural domains and asymmetry of amino acid residues in SSA-B/La nuclear protein. J Immunol 136: 3744–3749, 1986. 10. Choi BR and Salama G. Optical mapping of atrioventricular node reveals a conduction barrier between atrial and nodal cells. Am J Physiol Heart Circ Physiol 274: H829–H845, 1998. 11. Efimov IR, Huang DT, Rendt JM, and Salama G. Optical mapping of repolarization and refractoriness from intact hearts. Circulation 90: 1469–1480, 1994. 12. Garcia S, Nascimento JH, Bonfa E, Levy R, Oliveira SF, Tavares AV, and de Carvalho AC. Cellular mechanism of the conduction abnormalities induced by serum from anti-Ro/SSA-positive patients in rabbit hearts. J Clin Invest 93: 718–724, 1994. 13. Gottlieb E and Steitz JA. Function of mammalian La protein: evidence for its action in transcription termination by RNA polymerase III. EMBO J 8: 851–861, 1989. 14. Ho SY, Esscher E, Anderson RH, and Michaelsson M. Anatomy of congenital complete heart block and relation to maternal anti-Ro antibodies. Am J Cardiol 58: 291–294, 1986. 15. Kanai A and Salama G. Optical mapping reveals that repolarization spreads anisotropically and is guided by fiber orientation in guinea pig hearts. Circ Res 77: 784–802, 1995. 16. Lerner MR, Boyle JA, Hardin JA, and Steitz JA. Two novel classes of small ribonucleoproteins detected by antibodies associated with lupus erythematosus. Science 211: 400–402, 1981. 17. Mazel JA, El-Sherif N, Buyon JP, and Boutjdir M. Conduction abnormalities in a murine model for the clinical syndrome of congenital heart block. Circulation 99: 1914–1918, 1999. 18. McCue CM, Mantakas ME, Tingelstad JB, and Ruddy S. Congenital heart block in newborns of mothers with connective tissue disease. Circulation 56: 82–90, 1977. 19. Menon A, Silverman ED, Gow RM, and Hamilton RM. Chronotropic competence of the sinus node in congenital complete heart block. Am J Cardiol 82: 1119–1121, 1998. 20. Miranda E, Boutjdir M, Tseng CE, Didonato FD, Chan EKL, and Buyon J. Induction of antibodies reactive with SSA/ Ro-SSB/La and development of congenital heart block in a murine model. J Rheumatol 161: 5886–5892, 1998. 21. Nygren A, Fiset C, Firek L, Clark JW, Lindblad DS, Clark RB, and Giles WR. Mathematical model of an adult human atrial cell: the role of K⫹ currents in repolarization. Circ Res 82: 63–81, 1998. 22. O’Brien CA and Wolin SL. A possible role for the 60-kD Ro autoantigen in a discard pathway for defective 5S rRNA precursors. Genes Dev 8: 2891–2903, 1994. 24. Salama G, Lombardi R, and Elson J. Maps of optical action potentials and NADH fluorescence in intact working heart. Am J Physiol Heart Circ Physiol 252: H384–H394, 1987. 25. Scott JS, Maddison PJ, Taylor PV, Esscher E, Scott O, and Skinner RP. Connective tissue disease, antibodies to ribonucleoprotein and congenital heart block. N Engl J Med 309: 209–212, 1983. 26. Waltuck J and Buyon JP. Autoantibody associated complete heart block: outcome in mothers and children. Ann Intern Med 120: 544–551, 1994. Downloaded from http://ajpheart.physiology.org/ by 10.220.33.1 on May 6, 2017 nel internalization, degradation, cell death, and eventually fibrosis and calcification, as has been reported from the autopsies of affected children (14). Because cardiac myocytes do not undergo any significant mitosis after birth, affected cells are not replaced, and thus this may explain the irreversibility of the 3° AV block in human infants. The partial recovery of AV block reported in this study is likely due to the acute exposure of the antibodies, unlike chronic exposure during the pregnancy term. Altogether, these findings strongly suggest that maternal IgG are causally related to a number of electrophysiological abnormalities in the development of CHB. However, it should be emphasized that not all mothers with anti-SSA/RoSSB/La antibodies have affected children. This discordance may be related to undocumented electrophysiological effects or subclinical aspects of autoimmunerelated CHB indicating that additional factors are required to promote disease expression. In conclusion, the use of optical mapping techniques allows for the correlation of conduction disorders to specific anatomic structures in the heart because recordings could be mapped from multiple sites. We showed that CHB was associated with marked sinus bradycardia and conduction delays at the SA junction. These findings provide potential new insights into understanding the pathogenesis of CHB and raise the question of whether screening of infants from affected mothers for sinus bradycardia should be part of standard diagnosis. H1895