* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Notes on AB Structure II
Survey
Document related concepts
Transcript
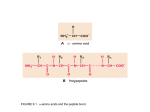
Immunology: Antibody Structure II (Montgomery) GENERAL Ig STRUCTURE: IgG is the Prototype 4 Amino Acid Chains: 2 light chains: κ or λ (~210aa long) 2 heavy chains: type determines Ig class (~420aa long) Disulfide Bonds: Interchain: link light and heavy chains (configuration varies) Intrachain: involved in chain folding and domains of the molecule (configuration fairly constant) Variable and Constant Regions: Variable: region near the N-terminus that varies in amino acid sequence Constant: o Light Chain: Cκ and Cλ o Heavy Chain: CH1, CH2, CH3 (δ,γ,α) and CH4 (μ and ε) Hinge Region: located between the CH1 and CH2 on heavy chains; provides segmental flexibility (allows molecule to interact with antigens) Carbohydrate Attachment Site: CH2 domain Subregions of the Molecule: o Fab: 2 per IgG; light chain + ½ heavy chain o Fc: 1 per IgG; 2 x ½ heavy chain o Fv: smallest fragment that retains site for reaction with Ag; 2 per IgG; made up of the variable regions of both light and heavy chains FRAGMENTATION OF THE MOLECULE: Reduction/Alkylation of Disulfide Bonds: Results in separation of the chains from one another o H chain has reduced (but not completely absent) binding capacity o L chain has NO binding capacity Conclusion: both H and L chain needed for full Ag binding capacity Papain Fragmentation: Results in 2 Fab fragments and 1 Fc fragment o Monovalent Fab fragments retain full Ag binding capacity o Fc portion did not bind Ag Pepsin Fragmentation: Results in 1 F(ab')2 and peptides o Divalent F(ab')2 retained full Ag binding capacity o Fc function totally lost, resulting in: Inability to fix complement Inability to bind cells with FcR Inability to cross placenta (IgG) IgG STRUCTURE: Monomeric 7S Heavy Chain Subclasses: γ1, γ2, γ3, γ4 Function: serum secondary response IgA STRUCTURE: Multimeric Varieties: Monomeric 7S Polymeric 10S Polymeric 11S (Secretory Form): requires 2 cells to produce sIgA o Plasma cell: produces L, H and J chains (MW 16,000) o Epithelial cell: produces secretory component (MW 70,000) Secretory Component: protects Abs from degradation Heavy Chain Subclasses: α1, α2 IgM STRUCTURE: Pentameric 19S Also includes a J chain Heavy Chain: μ (longer than IgG; contains CH4 domain) Function: serum primary response (evidence of recent infection); efficient agglutinator IgD STRUCTURE: Monomeric 8S Heavy Chain: δ (slightly larger H chain than IgG; consists of CH1, CH2 and CH3 domains as well as a partial domain) Location: mostly located on cell surface (not much free) IgE STRUCTURE: Monomeric 8S Heavy Chain: ε (longer than IgG; contains CH4 domain) Location: free or fixed to mast cell surfaces Function: immediate hypersensitivity ANTIBODY COMBINING SITE: Factors to Consider: Ag-Ab interactions are noncovalent Variation in capacity to bind Ag Number of Ab combining sites Gross architectural similarities Parameters Defining Site: Unique Amino Acid Sequence Defines Site: encoded by genome Dimensions of the Site: varies from Ab to Ab, but very small (Angstroms) Location of the Site: contacts residues within the variable regions of L and H chains (Fv) o Affinity Labeling: made us aware of the presence of hypervariable regions/complimentarity determining regions (CDRs) on both heavy and light chains o Variable Regions: Hypervariable regions/CDRs: come together to form the antibody combining site, which binds Ag Framework regions ANTIBODY HETEROGENEITY: Levels of Antibody Diversity: Species Isotypic: heavy chains differ in either class or subclass (ie. IgM vs. IgG; IgG γ1 vs. IgG γ2) Allotypic: variance among different individuals due to genetically determined amino acid insertions Insertions usually in the constant regions of the heavy and light chains Human allotype reagents come from patients receiving multiple transfusions Idiotypic: amino acid inserts in the variable regions of both the light and heavy chains Inserts can occur in both the hypervariable/CDR region and the framework residue Have a shared antigen binding specificity Importance: o Immune regulatory network o Vaccine development Vaccine Development Using Idiotypes: Ag injected into 1 Ab1 produced; injected into 2 Ab2 produced; injected into 3 Ab3 produced Ab2: anti-idiotype (reacts with Ab1) Ab3: anti-anti-idiotype (reacts with both Ab2 and Ag) Therefore, the injection of an anti-idiotype results in production of Abs against the Ag in question