* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download File
Homoaromaticity wikipedia , lookup
Marcus theory wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Discodermolide wikipedia , lookup
Asymmetric hydrogenation wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Metal carbonyl wikipedia , lookup
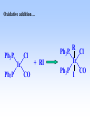
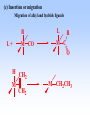
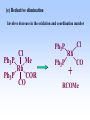
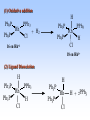
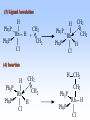
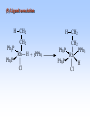
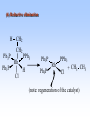
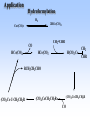
Homogeneous catalysis A+B Catalyst Catalyzed rxn proceeding through an intermediate C Heterogeneous Homogeneous Ea G Ea c a ta ly ze d A catalyst lower the activation Re a c ta nts G barrier for a transformation, Produc ts by introducing a new reaction Re a c tion Coordina te pathway. It does not change the thermodynamics!! Important catalyst properties * Activity: A reasonable rate of reaction is needed Turnover frequency (N) N = /[Q] Large turnover frequency – efficient catalyst * Selectivity: Byproducts should be minimized * Lifetime: It is costly to replace the catalyst frequently * Cost: The acceptable cost depends upon the catalyst lifetime and product value lifetime and product value Transition metal organometallic compounds Metal-carbon bond 18 en rule Organic compounds – Octet rule Organometallic – 18 electron rule * 18 valance electron – inert gas configuration Oxidation state method Neutral atom method Ligand Name Molecular Hydrogen: H2 Hydride H- Bonding Type H M Formal Charge Electrons donated 0 2 -1 2 H M-H Halide X- M-X -1 2 Amine, phosphine, arsine: NR3, PR3, AsR3 M-NR3 M-PR3 0 2 0 2 -1 2 -1 2 Carbonyl: CO Alkyl , Aryl M C O M-CR M-Ph M Alkene H2C CH2 CO OC Fe OC Fe is 4s23d6 = 8e CO each Co is neutral so Fe0 CO each CO donates 2 e = 10e 8e + 10e = 18e coordinately saturated Ph3P Ph3P Rh PPh3 Rh is s1d8 = 9e Cl since Cl is -1, Rh is +1 (the complex is neutral) 9e - 1e + 8e = 16e 4 ligands x 2e each = 8e therefore coordinately unsaturated Ph3P 16 electron complexes Ir OC Group 9 and 10 Exception to 16/18 electron rule V(CO)6 17 electrons W(CH3) 12 electrons Cl PPh3 9+1+2+2+2 = 16 Catalytic steps (a) Ligand coordination and dissociation Facile coordination of the reactant and facile loss of products. Coordinatively unsaturated - 16-electron complexes (b) Oxidative addition Non-bonding electron pair in the metal Coordinatively unsaturated Oxidation of metal by two units – Mn to Mn+2 Oxidative addition… Ph3P Cl Ir Ph3P CO + RI R Ph3P Cl Ir Ph3P I CO (c) Insertion or migration Migration of alkyl and hydride ligands L+ R L M CO M C R O H CH 2 M CH2 M CH2CH3 (d) Nucleophilic attack R C R 2+ L3Pd H R L3Pd OH2 C C OH R R C H R O L5M + - CO + OH L5M C OH L5M H + CO2 - (e) Reductive elimination Involves decrease in the oxidation and coordination number Ph3P Ph3P Cl Rh Ph3P Me COR CO Ph3P Cl Rh + CO RCOMe Wilkinson’s Catalyst PPh3 Ph3P Rh Cl PPh3 Tris(triphenylphosphine)rhodium(I) chloride square planar 16 electron species d8 configuration Geoffrey Wilkinson • Born July 14, 1921, Yorkshire, England Todmorden, •Received Ph.D from Cal studying with Glenn Seaborg Berkeley •First published compound in 1965 in Journal of the Chemical Society Chemical Communications •Nobel Prize in Chemistry 1973 (shared with Ernst Otto Fischer) for their pioneering work, performed independently, on the chemistry of the organometallic, so called sandwich compounds. Synthesis PPh3 Ph3P RhCl3 + 3 H2O + EtOH + Rh >4 PPh3 78 oC Cl PPh3 commercially available – Sigma Aldrich, Acros, etc. Ph3PO (1) Oxidative addition H Ph3P Rh Ph3P PPh3 Cl Ph3P + H2 Rh Ph3P PPh3 H Cl 16 en Rh+1 18 en Rh3+ (2) Ligand Dissociation H Ph3P Rh Ph3P PPh3 H Cl H Ph3P Rh Ph3P Cl H + PPh3 (3) Ligand Association H Ph3P Rh Ph3P H + CH2 CH2 Cl CH2 H Ph3P CH2 Rh Ph3P H Cl (4) Insertion H Ph3P CH2 CH2 Rh Ph3P H Cl H CH2 Ph3P CH2 Rh Ph3P Cl H (5) Ligand association H CH2 H CH2 CH2 CH2 Ph3P Rh Ph3P Cl H + PPh3 Ph3P Rh Ph3P PPh3 H Cl (6) Reductive elimination H CH2 Ph3P CH2 Rh Ph3P PPh3 H Cl Ph3P Ph3P Rh PPh3 Cl + CH3 CH3 (note: regeneration of the catalyst) WC IN A C T I O N Highly sensitive to the nature of the phosphine ligand Analogous complexes with alkylphosphine ligands are inactive Chiral phosphine ligands have been developed to synthesize optically active products. Applications * Laboratory scale organic synthesis * Production of fine chemicals * Synthesis of L-DOPA Used for the treatment of Parkinson’s diseases Synthetic route was developed by Knowles and co-workers at Monsanto Dr. William S. Knowles received Nobel prize in chemistry 2001 along with other two scientists. This reaction, developed by Knowles, Vineyard, and Sabacky, was used at Monsanto as a commercial route to the Parkinson's drug LDOPA. Metal Carbonyl Complexes M-CO CO as a ligand strong s donor, strong π-acceptor strong trans effect small steric effect CO is an inert molecule that becomes activated by complexation to metals Homoleptic Heteroleptic Group Formula Valence en Structure CO CO 6 Cr(CO)6 OC Cr CO OC CO 6 +12 = 18 7 Mn2(CO)10 7+10+1 = 18 8 Fe(CO)5 8+10 = 18 OC CO COCO OC Mn Mn CO OC CO OC CO CO OC Fe CO OC CO CO OC OC 9 Co2(CO)8 9+8+1= 18 CO Co CO Co OC C O C O CO Ni OC 10 Ni(CO)4 10+8 = 18 CO CO O M C s orbital serves as a very weak donor to a metal atom O C M CO-M sigma bond O C M M to CO pi backbonding O C M CO to M pi bonding (rare) Metal should be in low oxidation state ie en rich Synthesis Direct combination Ni(s) + 4CO(g) Ni(CO)4(l) 30oC and 1 atm CO Reductive carbonylation CrCl3(s) + Al(s) + 6CO(g) AlCl3(soln) + Cr(CO)6 (soln) AlCl3 and Benzene Re2O7(s) + 17 CO(g) 250oC, 350 atm CO Re2(CO)10(s) + 7CO2(g) Substituted carbonyls Oxidative Addition Concomitant oxidation of the metal and addition of new ligand(s) Fe(CO)5 + Br2 Fe0 Fe2+ Fe(CO)4Br2 + CO Reductive Carbonylation Metal halide and CO RhCl3.3H2O CO/EtOH 100 o Rh2(CO)4Cl2 Properties Reduction to form metal carbonylates Fe(CO)5 Na, THF 2- [Fe(CO)4] + CO Substitution Cr(CO)6 + Solv Cr(CO)5(solv) +PR3 Cr(CO)5(solv) + CO Cr(CO)5PR3 + solv [Cr(CO)5Solv] Cr(CO)5(PR3) Cr(CO)6(PR3)]# [Cr(CO)5(PR3)] O v C l o +S Cr(CO)6 R3 +P -CO Oxidation (CO)5Mn-Mn(CO)5 + Br2 2Mn(CO)5Br Oxidative cleavage of M-M bond Protonation Mn(CO)5-(aq) + H+(aq) HMn(CO)5(s) …more negative the anion, higher its Bronsted basicity … use in the synthesis of different organometallic [Mn(CO)5]- + CH3I [Mn(CH3)(CO)5] + I- Application Hydroformylation H2 Co2(CO)8 2HCo(CO)4 CH2=CHR CO HCo(CO)3 HCo(CO)4 H(CO)3Co CH2 CHR RCH2CH2CHO (CO)3Co C-CH2CH2R (CO)4CoCH2CH2R (CO)3CoCH2CH2R CO