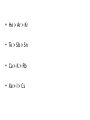* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Atomic Radius and Ionization Energy
Bremsstrahlung wikipedia , lookup
Ferromagnetism wikipedia , lookup
Wave–particle duality wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
Molecular Hamiltonian wikipedia , lookup
Hydrogen atom wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Electron scattering wikipedia , lookup
Chemical bond wikipedia , lookup
Auger electron spectroscopy wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Atomic orbital wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Electron-beam lithography wikipedia , lookup
Tight binding wikipedia , lookup
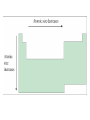
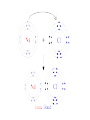
Periodic Trends Section 6.3 The Periodic Law states... Periodic Law • The pattern of properties within a period repeats as you move across a period from left to right… When elements are arranged in order of increasing atomic number, there is a periodic repetition of their physical and chemical properties Periodic Trends • Trend – a predictable change • Our focus will be on the main block elements • How do electron configurations help us explain many of the trends in properties observed? Trend in Atomic Radius • Measure the molecule that forms when two atoms of the same element combine • Atomic radius = half of the distance between the nuclei of the two atoms • Measured in picometers (1 pm = 10-12 m) Why does this trend occur? • 1. Changes in n- As the principal quantum number (n) increases, the outer electrons are farther from the nucleus, so the atoms are larger. • 2. Changes in Zeff – As the effective nuclear charge increases, outer electrons are pulled closer to the nucleus, so the atoms are smaller. • (Zeff = the positive charge felt by an electron) 1. Down a group, n dominates • Elements have one more energy level of core electrons • These SHIELD the outer electrons • Atomic radius generally INCREASES in a group from top to bottom 2. Across a period, Zeff dominates • Moving across a period, electrons are added to the same outer level • Shielding by inner electrons does not change • Zeff increases and outer electrons are pulled closer • Atomic radius generally DECREASES in a period from left to right Try the following… • Put in order of decreasing atomic size • Ca, Mg, Sr • K, Ga, Ca • Br, Rb, Kr • Sr, Ca, Rb • Sr > Ca > Mg • K > Ca > Ga • Rb > Br > Kr • Rb > Sr > Ca Ions • Atom or group of atoms that has a positive or negative charge • Form when electrons are transferred between atoms • Metals tend to lose electrons, forming cations • Nonmetals gain electrons, forming anions Trends in Ionization Energy • Energy needed to remove an electron from an atom • Energy needed to remove the first electron from an atom is the FIRST IONIZATION ENERGY • Produces cation with 1+ charge • First ionization energy tends to decrease from top to bottom in a group and increases from left to right across a period Why does this trend occur? • As atomic size increases down a group, Zeff has a smaller effect on the electrons in highest level • Shielding Effect • Less energy is needed to remove electron from the energy level making first ionization energy lower Across a period… • Zeff increases and shielding effect remains constant • Increase in the attraction the nucleus has for an electron • More energy is needed to remove an electron from the atom and first ionization energy is higher Try these… • Put the following in order of decreasing IE1 • Kr, He, Ar • Sb, Te, Sn • K, Ca, Rb • I, Xe, Cs • He > Ar > Kr • Te > Sb > Sn • Ca > K > Rb • Xe > I > Cs