* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download a PDF of this article. - Journal of Invasive Cardiology
Cardiovascular disease wikipedia , lookup
Saturated fat and cardiovascular disease wikipedia , lookup
Heart failure wikipedia , lookup
Remote ischemic conditioning wikipedia , lookup
Cardiothoracic surgery wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Electrocardiography wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Cardiac surgery wikipedia , lookup
Drug-eluting stent wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
History of invasive and interventional cardiology wikipedia , lookup
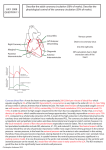
Original Contribution Review Myocardial Venous Drainage: From Anatomy to Clinical Use Dario Echeverri, MD, Jaime Cabrales, MD, Alejando Jimenez, MD Abstract: The heart’s venous drainage system has been the object of observations and research for several centuries. Its anatomic and histological characteristics, as well as its distribution and architecture, make the cardiac venous system a uniquely organized structure within the organism. An understanding of its physiology helps in comprehending some of the mechanisms by which, under special conditions, the myocardium tolerates ischemia. The heart’s venous system has become important today as a route for administering pharmacologic therapy, gene therapy, growth factors, and stem cells to the myocardium. Access through the coronary sinus is a common practice in modern electrophysiology. . 3 se 01 lU 2 na MP so H er ht r P rig Fo opy J INVASIVE CARDIOL 2013;25(2):98-105 retrograde perfusion for maintaining nutritional supply of the myocardium during periods of anoxia or cardioplegia has been routinely used in cardiovascular operating rooms. Studies of the heart’s vascular anatomy have focused mainly on the arterial segment with marked emphasis on the coronary arteries and myocardial capillaries; in contrast, the cardiac venous system has traditionally been forgotten. The studies described in the literature in the last decades are very scarce and have been limited to anatomy and topography of the CS and its related veins. In this sense, the new requirements for diagnosis and treatment in current cardiologic clinical practice require crossing the frontier, making the use of selective CS catheterization techniques a routine practice since the year 2000. The advances in diagnostic cardiac images and the improvements in angiography have permitted a better visualization of the venous segment of the cardiac circulation, facilitating the recently published angiography and computed tomography descriptions. The presence of accessory pathways of the conduction system in the vicinity of the CS has been shown since 1978, which causes electrophysiology groups to be more familiar with this structure and frequently requires selective catheterization of the same as part of diagnostic or therapeutic studies. The purpose of this document is to review generalities of the coronary venous system anatomy and the techniques used for its selective catheterization. It is motivated by the appearance of innovative percutaneous procedures that require intervention of the venous system, which has generated a growing interest in methods for accessing the heart’s venous circulation for coronary or structural interventionalism in the area of hemodynamics or for stimulation or therapeutic ablation in the area of electrophysiology. In patients where traditional techniques for myocardial revascularization are not feasible, procedures based on catheterization of coronary veins and retroperfusion (which can supply the minimal metabolic needs), have been proposed, arising as alternative treatment modalities. In addition, the use of the coronary venous system has allowed regional administration of drugs, cells, and genes for protecting and regenerating the sick myocardium. C Key words: ablation, arrhythmias, catheterization, circulation, veins Advances in medicine have allowed the development of useful diagnostic tests for recognizing and evaluating viable myocardial territory, which have decreased oxygen consumption to a minimum in order to maintain minimal cellular function and inhibit their contractile activity. The available treatment alternatives, which include pharmacologic therapies, coronary bypass surgeries, and various percutaneous procedures, have allowed the recovery of this viable and ischemic myocardium, significantly impacting the recurrence of ischemic symptoms, functional capacity, quality of life, and, ultimately, the survival of patients with ischemic coronary disease. We must remember the great lability of these cells with small changes in oxygen concentration and the importance the venous system assumes in maintaining cellular homeostasis. Over a century ago, in 1898, Pratt1 first described the retrograde myocardial perfusion techniques that protect hypoperfused myocardial cells through catheterization of the coronary sinus (CS) and the veins related to the ischemic territory. Pratt, a physiologist, was the first to publish this experimental technique with results in animal models. However, his results lacked acceptance at the time, and unfortunately, were practically forgotten. It was Beck et al, in 1948,2 who rediscovered this simple, but marvelous idea, resuming part of Pratt’s research and perfecting the model with results that led to recommending its application in clinical practice. In the last decades, From the Fundacion Cardioinfantil, Bogota, Colombia. Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Echeverri is on the Medtronic speaker’s bureau and board, and is a consultant for Biotoscana. The other authors report no conflicts of interest regarding the content herein. Manuscript submitted June 29, 2012, provisional acceptance given July 26, 2012, final version accepted August 8, 2012. Address for correspondence: Dr Dario Echeverri, FACC, Fundación Cardioinfantil, Bogota, Colombia, Calle 163 #13b – 60 Hemodinamia, Bogota, Colombia. Email: [email protected] 98 Cardiac Venous System Knowledge of epicardial venous anatomy is essential for the success of CS and cardiac vein catheterization. The venous anatomy of the heart and its common variants has been described in detail in autopsies and in vivo studies,3 where the majority of blood returns to the heart through the epicardial coronary veins, then progressively joins into larger conduits and ultimately flows into the right atrium through the CS. Tests carried out on coronary venous system molds show that the myocardial veins flow perpendicular to the surface of the ventricles to empty into the epicardial veins4 (Figure 1). The Journal of Invasive Cardiology® Myocardial Venous Drainage: From Anatomy to Clinical Use . 3 se 01 lU 2 na MP so H er ht r P rig Fo opy Figure 1. Schematic representation of the distribution pattern of the cardiac venous system and its tributary vessels. Modified from Lüdinhhausen MV. The venous drainage of the human myocardium. In: Advances in Anatomy, Embryology, and Cell Biology. Heidelberg, New York. Springer. 2003:4. The course of the anterior interventricular vein is parallel to the anterior descendent artery and flows adjacent to it over the interventricular septum toward the base of the heart, where it turns laterally toward the circumflex artery to become the great cardiac vein. The anterior interventricular vein has been analyzed using imaging techniques, and its diameter is similar to that of the artery. The great cardiac vein usually gathers affluents from the left ventricular surface through the anterior interventricular vein, a left marginal vein and a posterior left vein.8 In the inferior wall, at the atrioventricular sulcus, the great cardiac vein flows into the CS. The middle cardiac vein can be seen in the posterior interventricular sulcus, often together with the posterior descendent artery and also flows directly into the CS (Figure 2). Recently, after detailed angiographic studies, several groups have tried to describe the coronary venous anatomy;9 the number and location of the cardiac veins turned out to be variable in these patients. In a study by Meisel et al,10 the anterior interventricular vein and the middle cardiac vein were visible in 85 of 86 patients. However, the anatomy of the lateral marginal vein is more variable, present in 71 of 86 patients. C Transverse sections of the left ventricular heart wall have shown three venous vascularization zones, which, described from epicardium to endocardium, are: (1) external epicardial zone, which includes a thin external layer, approximately 1 mm thick, over the walls of the left ventricle; (2) middle subepicardial zone, which together with the external zone makes up more than 70% of the ventricular wall and the adjacent interventricular septum; and (3) internal subendocardial zone, which makes up the rest of the ventricular wall and interventricular septum.5 In the right ventricular walls, only two zones have been identified: (1) external epicardial zone over the external layer of the right ventricle; and (2) thick internal zone, which is the sum of the middle subepicardial and subendocardial zones. Based on these findings, anatomists, physiologists and cardiologists have divided the venous system of the cardiac circulation in three parts: (1) greater cardiac venous system (major – GCVS); (2) Smaller cardiac venous system (minor – SCVS); and (3) intramyocardial sinusoid system.6,7 Venous filling of all the ventricular wall zones is dependent on perfusion pressure. Pressures below 50 mm Hg allow filling only of external veins and the middle zone, while pressures above 80 mm Hg allow venous filling of all myocardial ventricular zones.5 Greater Cardiac Venous System (GCVS) The vessels of this system are located within the subepicardial layer with extensions over the atrial and ventricular surface. Frequently, its veins do not follow the topographic borders marked by the coronary arteries. The GCVS is made up of several intercommunicating parts: 1. The CS and its ventricular tributary veins 2. The anterior cardiac venous system 3. The superior interventricular septum veins 4. Left atrial veins 5. Mediastinal tributary veins to both atria 6. Right atrial veins Vol. 25, No. 2, February 2013 Lesser Cardiac Venous System In mammal’s hearts, there are multiple small, thin, vascular connections between the coronary arteries and the heart chambers, made up of canals, lacunae, and sinusoids located in the subendocardial layers of both the atrial and ventricular cavity walls. This system has been described since 1928 by Wearn et al11 and later confirmed by observations and descriptions of other authors.12,13 Numerous orifices of small vessels belonging to the LCVS are found, especially in the septal walls and the lateral wall of the right ventricle, as well as in the left atria.14 The presence of small venous vessel ostia has also been described in the surface of papillary muscles. The vessels of the LCVS are classified into four types, according to the descriptions of different authors:15,16 1. Sinusoidal vessels, which are the smallest veins (venules); connect the intramural veins with subendocardial sinusoids and drain the internal layers of the atrium and the ventricular myocardium. 2. Venoluminal vessels, which connect the intramural venous network with the atria or ventricles. 3. Arterio-sinusoidal vessels, which connect thin arteries or arterioles with the sinusoidal spaces in the internal layers of the atria and ventricles. 4. Arterio-luminal vessels, which connect small arteries or arterioles with atria or ventricles. Through microanatomy and histology techniques, using injected human heart specimens and electronic microscopy, it has been shown that the vessels that make up this system are of a highly variable form (canals, sinusoids, tree, etc), number, size, and length (on average, 15 mm). They go in all directions, without orientation and achieve connections with veins from the middle layer. Histologically, these vessels do not have a middle muscular layer. They are made up of a layer of endothelial cells that are continuous with the endothelium of the cardiac cavities.17 The vessels that make up the LCVS are able to collect a great amount of venous blood and they supply sufficient nutrients 99 ECHEVERRI, et al. . 3 se 01 lU 2 na MP so H er ht r P rig Fo opy Figure 2. Angiographic image of the coronary sinus (CS). Catheter located in the CS. Manual injection of contrast medium for retrograde filling. (A) Right anterior oblique 10° projection. (B) Left anterior oblique 30°, Cranial 10° projection. C Figure 3. Angiographic image of the coronary sinus (CS) and its tributaries: postero-lateral branches and middle cardiac vein. Catheter located in the CS. Manual injection of contrast medium through a balloon-catheter for retrograde filling to guide electrode placement for biventricular pacing. Anteroposterior view. from the ventricular cavities to the myocardium to be able to maintain rhythmic contractions for long periods of time. They are able to store close to 30% of the cardiac venous flow.18 Coronary Venous Sinus The CS is a superficial vascular structure located in the sulcus between the left atrium and the left ventricle. It is defined as the conduit of blood which is a continuation of the greater cardiac vein, extending from the greater cardiac vein valve to its ostium in the right atrium (Figure 3). The CS flows into the right atrium through the Thebesian valve. Although it has traditionally been considered simply a conduit of venous return 100 to the right atrium, a close examination reveals that its structure is similar to the other cardiac chambers, having endocardium, myocardium, and a specific conduction system that is continuous with that of the right atrium. These findings suggest that the CS may also play a role in the normal and abnormal electrical conduction of the heart.19 The CS is the most constant structure in the cardiac venous system. Although it has many variations in position, form, length, and diameter, it has been described as being up to 40 mm long in an 80 kg individual (in 75% of cases it is between 30 and 50 mm long) and 10 mm in diameter.20 It is cone shaped or cylindrical and its size has been equated to that of the middle phalange of the fifth finger of a healthy adult (Figure 2). In pathologic conditions, such as heart failure and ventricular hypertrophy, the CS may grow several times its size. It is found in the posterior portion of the coronary sulcus in the diaphragmatic (posterior) wall of the heart. It stretches from the left ventricular margin, from the left atrial oblique vein (vein of Marshall) up to the orifice in the right atrium, in the area known as the “crux of the heart.”21 The CS receives most of the ventricular epicardial veins. The confluence of the GCVS and the CS occurs at a terminal point delimited by the left atrial oblique vein. It then descends toward the posterior wall. The atrial ostium of the CS is frequently oval shaped and provides a valve with a concave edge. In some cases, this valve is absent (19% of specimens), and in others it is completely round, septal, semilunar, or cribriform. In rare cases, it is severely dilated or aneurysmic, resembling a compact intrapericardial tumor. Thebesian Venous System The first vertebrates feed the myocardium by simply absorbing blood from the lumen of their ventricles. This simple diffusion is the classic method of nutrition and waste elimination in inferior organisms that lack vascular systems.22 At the The Journal of Invasive Cardiology® Myocardial Venous Drainage: From Anatomy to Clinical Use C . 3 se 01 lU 2 na MP so H er ht r P rig Fo opy Figure 4. CT angiography images that show the relationship of the coronary sinus to the epicardial blood. (A) Cranial view that assesses the relationship with the anterior descending artery. (B) Oblique view where the circumflex artery is associated with the coronary sinus. (C) Lateral view where the coronary sinus is seen in its greatest extent in the atrialventricular groove and its relation to the circumflex artery. In 1951, Tori26 was the first to describe the retrograde injection of contrast media to the myocardium through the heart’s CS. Since then, descriptions have been made of coronary venous retrograde perfusion preserving the myocardium during experimental ischemic coronary occlusion and it has been used clinically for administering oxygenated blood to the ischemic myocardium during unstable angina27,28 or high-risk percutaneous transluminal coronary angioplasty (PTCA),29-31 and in cardiac surgery,32 showing an improvement in ischemia and reducing episodes of chest angina. Retrograde percutaneous arterialization of coronary veins in patients unsuited for conventional myocardial revascularization has been previously described.33 The selective delivery of genes, growth factors, and stem cells to the myocardium through the coronary veins has shown higher success rates than intra-arterial or systemic delivery, supporting this as a novel use of the heart’s venous system.34-37 It has also been used by electrophysiologists as an electrode insertion route for left ventricular stimulation in cardiac resynchronization therapy,38 ablation with radiofrequency catheters, arrhythmia mapping,39 and defibrillation.40 beginning of the 17th century, Raymond Vieussens (16351713) and Adam Christian Thebesius (1686-1732) discovered the vasa cordis minima, known today as the Thebesian veins. These veins are part of the LCVS. Careful anatomic analyses have identified three distinct forms of these veins.3,23,24 The first type of venous drainage from the Thebesian veins consists of the capillary bed toward the ventricular cavity.23 In the second type, the arterioluminal vessels drain blood directly from the arteries toward the ventricles without crossing the capillary beds, thus having a greater diameter. In the third type, the venoluminal vessels form direct connections with the coronary veins, carrying the blood from these vessels to the ventricular cavities.25 Most Thebesian vessels in the ventricles appear to be of a venoluminal nature.3 The presence of an extensive Thebesian venous network, which in a healthy state contains 5%10% of venous return, is an alternative route for myocardial venous drainage. It has been determined that the Thebesian veins are able to carry out the majority of venous return in situations where the epicardial coronary veins are compromised. Coronary Venous System: An Alternative Myocardial Access Route The coronary venous system has been studied with great interest recently as an alternative route for accessing the myocardium. There are three conditions in which an understanding of the venous system can lead to useful clinical interventions. First of all, the use of percutaneous techniques for allowing retroinfusion of oxygenated (arterialized) blood in the coronary veins of patients considered to be unsuited for conventional revascularization procedures. Second, the regional application of therapeutic agents such as cardio-protective drugs, cells, or gene vectors. And last, the use of the coronary venous system as an access route to the myocardium for electrophysiology procedures. Vol. 25, No. 2, February 2013 Access to the Coronary Venous System Percutaneous cannulation of the CS and some tributary coronary veins, as well as the possible applications of this technology, have been described for some time.41 Different catheter types have been used to properly gain access to the coronary veins through the CS. Access has been reported through the right and left subclavian veins, the right internal jugular vein, and the femoral veins. Katritsis et al42 reported success in cannulation of the CS through the femoral vein using a modified 6 Fr Judkins JL5 catheter. Other authors report access to the CS with a left-type Kids Health catheter in 15 patients with an average cannulation time of 3.34 ± 1.9 minutes.43 Coronary complications of CS cannulation have been reported in a small percentage, which include arrhythmia, dissection, and perforation with hemopericardium.44 Retrograde Venous Coronary Perfusion The CS and its affluents provide an alternate access route to the myocardium in the presence of total occlusions or severe stenosis of the coronary arteries. The first studies by Pratt,45 Beck,31 and Batson and Bellet46 documented the possible benefit of reverse flow in the coronary venous system for oxygenation and nutrition of the myocardial cells. By perfusing the CS, Pratt was able to maintain cardiac contractions for 90 minutes in a devascularized feline heart model. Gross et al47 reported an attempt to increase coronary perfusion while partially occluding the CS in animals. These animals tolerated anterior descendent artery ligation better than control animals that did not have partical CS occlusion. The multiple descriptions that rule out coronary venous system compromise due to atherosclerosis27 encourage even more research of the coronary venous system as an alternative system for maintaining nutritional flow to the myocardium in patients with severe involvement of arterial circulation. Prior to the advent of bypass surgery, surgeons experimented with arterializations of the coronary venous system. Beck et al31 refined this procedure during the 1940s. In an operation known as the Beck II procedure, they would first obstruct the CS using ligation. After 10 days, during which 101 ECHEVERRI, et al. for PTCA. In the 1980s, retroperfusion of the CS was used as a way to protect the myocardium during angioplasty in this group of patients. The technique was able to reduce both anginal symptoms during PTCA as well as the time to appearance of ischemia as seen in ST-segment changes following balloon inflation.30 However, in spite of these results, the use of retroperfusion of the CS continues to be limited. The main reason for its underutilization seem to be that the retroperfusion equipment has technical limitations and is slow to configure; in addition, it is technically impossible in 10%-20% of patients. Also, the degree of myocardial protection, although of some benefit, was proven to be limited and the technique was only tested in anterior descending artery interventions. Local Drug Delivery Numerous studies have researched the efficacy of retrograde perfusion of pharmacologically active agents to the myocardium through the coronary veins. In intact, non-ischemic myocardiums, the medication concentration following retroinfusion is similar to that achieved when the medications are administered intravenously (IV). However, in the context of myocardial ischemia, retroinfusion achieves higher levels of concentration of the medication in the tissue than when it is administered IV, with the advantage of having lower peak systemic concentrations and fewer systemic side effects.57-60 This increase in tissue penetration is probably due to an improved low-pressure access to the capillary bed and diminished washout of the drugs due to the decreased anterograde blood flow. Thrombolytic agents, when administered directly in the coronary veins, act faster, improve functional recovery, and reduce the size of the infarct compared with systemic administration.37 C . 3 se 01 lU 2 na MP so H er ht r P rig Fo opy the CS had fibrosed, an arterial or venous conduit was placed between the aorta and the CS. The initial experimental work on this procedure was carried out on dog animal models. This revealed two important physiological characteristics: the CS could tolerate arterial pressure for a long period of time, and there was not a broad fistula effect following CS arterialization. Beck’s focus was termed “global retroperfusion” as the method for carrying oxygenated blood to the whole coronary venous system. Beck perfected his experimental technique (Beck II procedure), and in 1954, he and Leighninger32 reported a series of 200 patients who had undergone this procedure. However, they found that the permanent obstruction of coronary venous drainage was associated with specific problems, including hemorrhage, fibrosis, and myocardial edema. A second means of venous revascularization, the selective method, in which blood flow is reversed only in precise ischemic areas caused by arterial disease, was proposed by Arealis.48 Using this method, only a small portion of venous drainage is reversed, with the epicardial and Thebesian venous systems, and other territories continue to function normally. Various groups have perfected these procedures throughout the following years with encouraging results.28,49,50 With the advent of bypass surgery, interest in this technique dwindled; ongoing studies were abandoned and new studies on this topic were relegated. The advent of synchronized diastolic coronary retroperfusion allowed intermittent venous drainage of the myocardium, which is produced during retrograde perfusion. This technique has been shown to decrease pain episodes in patients with unstable angina51 and reduce complications in patients undergoing high-risk PTCA.29,52,53 It is clear that with the new revascularization techniques and the evolution of pharmacologic therapy generating an increased life expectancy in patients with coronary disease, the number of patients considered poor candidates for myocardial revascularization using conventional techniques is increasing and this has renewed interest in the techniques that use coronary veins to carry oxygenated blood to ischemic myocardial zones. A novel percutaneous procedure for retrograde arterializations of the anterior coronary veins has been developed and carried out recently in man.33,54 This technique has been used in patients with anterior descending artery occlusion in whom the anterior ventricular vein has been arterialized. The procedure was carried out using specially designed catheters which allow passage of a 0.014˝ guide and subsequent release of a stent that connects the artery and the vein. However, according to the authors, the procedure has been limited by technical problems.55 The percutaneous bypass between the ventricle and a coronary vein is being evaluated as a novel technique for patients who are not suitable candidates for revascularization. The procedure consists in implanting a polytetrafluoroethylene-covered stent directly between the left ventricular cavity and the superimposed coronary vein, in order to provide systolic flow of arterial blood in the coronary vein and also allow diastolic drainage of the vein in the ventricular cavity. The procedure has proven to be technically viable and able to maintain cardiac function in the presence of an occluded coronary artery. Clinical trials are currently under development.56 Patients with poor left ventricular function and large areas of ischemic myocardium are considered high-risk patients 102 Delivery of Genes, Growth Factors and Stem Cells The genomic and proteomic revolution continues advancing, and the underlying molecular biology of several cardiovascular pathologies is increasingly well understood. This understanding, in turn, leads to an increase in the number of possible molecular therapies available. The success of these treatments depends on many factors, among which is the ability to be delivered adequately to the target tissues. Some agents have shown bioactivity when administered systemically. However, it is assumed that most agents are more effective and better tolerated if they are delivered locally. The delivery of genes to the heart has been attempted using viral and non-viral vectors. Adenovirus is the viral vector that has proven most effective in vivo; however, adverse immune and inflammatory responses may limit its use. Direct injection of vectors based on plasmids to the myocardium has been reported, although the level of gene expression with this method is relatively low.36 The delivery of genes to the myocardium by retrograde venous perfusion in a porcine model has been reported34,35 and proved superior to percutaneous and surgical gene transfer.61 Penta et al62 showed a plasmid gene delivery vector, hDel-1, known to have angiogenic activity in angiogenesis trials when it is administered to the myocardium through the coronary veins. It was found in high concentrations in the myocardium, but is absent or at a much lower concentration in peripheral tissues. The Journal of Invasive Cardiology® Myocardial Venous Drainage: From Anatomy to Clinical Use in some patients, and for this reason, CS endo-epicardial ablation is an important step during radiofrequency ablation for patients with persistent AF today.80 The ligament of Marshall as an anatomical structure is a remnant of the left superior vena cava and contains nerve fibers with significant electrical conduction properties, connected to the CS muscular tissue in its proximal portion and with the left pulmonary veins in its middle portion. The vein of Marshall has been a focus point during ablation of paroxysmal AF in young patients, as this structure is an important trigger of arrhythmic events in up to 20% of these cases.81 Knowledge of the exact anatomical location of the CS ostium is paramount during ablation of AV nodal re-entrant tachycardia (AVNRT), as it helps delineate the anatomical boundaries of the triangle of Koch, which is made up in its posterior portion by the tendon of Todaro, anteriorly by the septal cusp in the tricuspid ring and inferiorly by the CS ostium.82 The middle portion of this anatomic triangle contains the compact AV node. In patients with AVNRT, there are at least two (and at times multiple) pathways with different conduction properties that constitute the necessary circuit for this re-entrant arrhythmia (classically labeled as slow-fast for typical AVNRT and fast-slow or slow-slow for atypical AVNRT). The slow pathway, which is usually located at the base of this triangle, is the preferred target of radiofrequency modulation or cryoablation for treating this arrhythmia, as the risk of complete heart block using this approach is <1%. Lastly, the coronary sinus provides direct access to the cardiac venous system as a potential approach during mapping and ablation of ventricular tachycardias originating from an epicardial substrate. By placing electrode catheters into the coronary sinus, electrical mapping of low-amplitude diastolic potentials and early local electrograms can guide successful VT ablation in areas of difficult endocardial access such as the mitral annulus (via the greater cardac vein) or the left ventricular summit, at the junction between the greater cardiac and the anterior interventricular veins.83-85 C . 3 se 01 lU 2 na MP so H er ht r P rig Fo opy Growth factors (GF) with angiogenic properties, such as fibroblast basic growth factor (FGF-2) and vascular endothelial growth factor (VEGF), induce growth of collateral coronary vessels.63 Various methods for introducing these GFs to the myocardium have been studied, such as direct injection to the myocardium and coronary artery infusion.64,65 Von Degenfeld et al66 reported the selective and regulated use via venous coronary retroinfusion of FGF in a porcine model of chronic ischemia. The venous delivery of GF has been favorably compared with intracoronary infusion and has also led to greater levels of meshing of the tissue with FGF-2 and has improved coronary perfusion through collateral vessels. Recent studies have emphasized stem cell transplant as an emerging treatment for patients in end-stage heart failure.67,68 The two main methods for delivering stem cells to the myocardium have been researched: these are direct (through intramyocardical injection)69 and intracoronary infusion.70 Both methods have proven to be effective. However, it has been shown that cells injected directly into the heart to form small islets may be isolated from native myocardium, and intracoronary infusion may not allow the cells to reach the ischemic myocardium. Suzuki et al(37) reported a rat model of retrograde venous perfusion of stem cells in the myocardium. This infusion route has been proven to allow cellular diffusion to all layers of the myocardium with minimal adverse effects seen during or following the retrograde infusion. Coronary Venous System in Cardiac Elecrophysiology The knowledge of coronary venous anatomy is of vital importance for the practice of modern electrophysiology. The venous system has become a crucial access route for the invasive management of pathologies, such as advanced cardiac failure, atrial fibrillation, and some supraventricular and ventricular tachycardias. The CS allows percutaneous endocardial access to posterolateral veins that drain the left ventricle, through which guides and catheters can be advanced for placing electrodes used in cardiac resynchronization therapy through programmed biventricular stimulation in patients with advanced heart failure. This improves their functional class and decreases ventricular dyssynchrony which, in turn, improves the effectiveness of myocardial contractions, increases forward cardiac output and decreases regurgitant mitral volume (Figure 3).40,71-74 The CS approach is also useful in managing atrioventricular (AV) re-entrant arrhythmias, being routinely used as an alternative approach in the ablation of left accessory pathways, as the majority of these anomalous connections tend to have epicardial fibers and insertions which on occasion cannot be eliminated through conventional endocavitary ablation.75-77 In some cases, AV re-entrant tachycardias that use the ligament of Marshall as a re-entry mechanism have been successfully eliminated by catheter ablation through the CS and the vein of Marshall.78 In addition, some CS fibromuscular tissue bands have electrical conducting properties, and cases of atypical atrial flutter have been described in patients following pulmonary vein isolation.79 On occasion, these CS muscular fibers can be the substrate that triggers and perpetuates atrial fibrillation (AF) Vol. 25, No. 2, February 2013 Venous System in Valvular Interventions With the growing interest in structural interventions and the development of percutaneous techniques for the treatment of valvular pathologies through percutaneous routes, the cardiac venous system, and especially the coronary sinus, is attractive because it is a structure located parallel to the posterior portion of the mitral ring and surrounding up to two thirds of the ring, making it possible to implant devices via this route with the capacity to shorten the length of the coronary sinus and in this way carry out a valvular annuloplasty. Early animal studies have shown that devices implanted through the coronary sinus can reduce the circumference of the mitral ring in normal mitral valve models or induced mitral insufficiency models in induced dilated cardiopathy with a rapid pacemaker.86 In a generic way, these devices seek to create a tension or constriction of the sinus, which is transmitted to the mitral valvular apparatus and especially to the valvular ring. Currently, there are initial experiences in humans with the Cardiac Dimensions Carillon (Cardiac Dimensions), Edwards Monarc System (Edwards Lifesciences), and the Viacor PTMA system (Viacor) devices. 103 ECHEVERRI, et al. Conclusion The knowledge of cardiac venous drainage, in spite of centuries of study, has been neglected. Only in the last decades has interest been shown in the coronary veins as a possible myocardial access route for catheter interventions and delivery of therapeutic agents. In spite of the early promise of retrograde perfusion in patients undergoing high-risk PTCA, this technique is not routinely used today. However, retrograde arterialization of coronary veins is a new percutaneous treatment with great potential for patients who “do not have the option” of myocardial revascularization due to severe symptomatic coronary disease. The use of coronary veins as a conduit for therapies such as gene vectors and stem cells is garnering greater attention and will play an important role in the development of these treatments. References 1. Pratt FH. The nutrition of the heart through the vessels of Thebesius and the coronary veins. Am J Physiol. 1898;1(1):86-103. 2. Beck BC. Revascularisation of the heart. Ann Surg. 1948;128(4):854-864. 3. Ansari A. Anatomy and clinical significance of ventricular Thebesian veins. Clinical Anatomy. 2001;14(2):102-110. 4. Gensini GG, Di Giorgi S, Coskun O, et al. Anatomy of the coronary circulation in living man. Circulation.1965;31:778-784. 5. Ovenfors CO. Venous communications between the cardiac veins and the large venous trunks in the superior parts of the mediastinum. Acta Radiol. 1956;46(3):518-522. 6. Lindeau KF, Romaniuk P. Anatomie des koronarsystems. In: Warnke H, ed. Chirurgie der Koronaren Herzerkraunkungen. Barth, Leipzig. 1983:29-31. 7. Mohl W. Surgical coronary sinus interventions for myocardial protection. In: Mohl W, ed. Coronary Sinus Interventions in Cardiac Surgery. 2nd edition. Williams & Wilkins, Baltimore, London. 2000:97-133. 8. Kawashima T, Sato K, Sato F. An anatomic study of the human cardiac veins with special reference to the drainage of the great cardiac vein. Ann Anat. 2003;185(6):535-542. 9. Gilard M, Mansourati J, Etienne Y, et al. Angiographic anatomy of the coronary sinus and its tributaries. Pacing Clin Electrophys. 1998;21(11 Pt 2):2280-2284. 10. Meisel E, Pffeiffer D, Engelmann L, et al. Investigation of coronary venous anatomy by retrograde venography in patients with malignant ventricular tachycardia. Circulation. 2001;104(4):442-447. 11. Wearn JT. The role of the Thebesian vessels in the circulation of the heart. J Exper Med. 1928;47(2):293-316. 12. Hammond GL, Austen WG. Drainage patterns of coronary arterial flow as determined from the isolated heart. Am J Physiol. 1967;212(6):1435-1440. 13. Vajda J, Tomscik JR, van Doorenmaalen WJ. Connections between the venous system of the heart and the epicardial lymphatic network. Acta Anat. 1972;83(2):262-74. 14. Schlant RC, Sonnenblick EH. Normal physiology of the cardiovascular system. In: Schlant RC, Alexander RW, eds. Hurst’s The Heart. 8th edition. McGraw Hill, New York. 1994:113-151. 15. Tschabitscher M. Anatomy of the coronary sinus: In: Mohl W, Wolner W, Glogar D, eds. The Coronary Sinus. Steinkopff Darmstadt, Springer, New York. 1984:8-25. 16. Ansari A. Anatomy and clinical significance of ventricular Thebesian veins. Clin Anat. 2001;14:102-110. 17. Robb JS. Comparative Basic Cardiology. Grune & Stratton, New York, London. 1965:123-140. 18. Hochberg MS, Austen WG. Selective retrograde coronary venous perfusion. Ann Thorac Surg. 1980;29(6):578-588. 19. Barcelo A, De la Fuente LM, Stertzer SH. Anatomic and histologic review of the coronary sinus. Int J Morphol. 2004;22:331-338. 20. McAlpine WA. Heart and Coronary Arteries. Springer, Berlin, Heidelberg, New York. 1975:179-209. 21. Maric I, Bobinac C, Ostojic L, Petrovic M, Dujmovic M. Tributaries of the human and canine coronary sinus. Acta Anat. 1996;156:61-69. 22. Hochberg MS, Folkman J. Mechanism of size limitations of bacterial colonies. J Infect Dis. 1972;126(6):629-635. 23. Grant R, Viko LE. Observations on the anatomy of the Thebesian vessels of the heart. Heart. 1929;15:103. 24. Wearn JT, Mettier SR, Klump TG, Zschiesche LJ. The nature of the vascular communications between the coronary arteries and the chambers of the heart. Am Heart J.1933;9:143-164. 25. Hood WB Jr. Regional venous drainage of the human heart. Br Heart J. 1968;30(1):105-109. 26. Tori G. Radiological visualization of the coronary sinus and coronary veins. Acta Radiologica. 1951;36(5):405-410. 27. Bhayana JN, Olsen DB, Byrne JP, et al. Reversal of myocardial ischaemia by arterialisation of the coronary vein. J Thorac Cardiovasc Surg. 1974;67(1):125-132. 28. Gardner RS, Magovern GJ, Park SB, et al. Arterialisation of coronary veins in the treatment of myocardial ischemia. J Thorac Cardiovasc Surg. 1974;68(2):273-282. 29. Hajduczki I, Kar S, Areeda J, et al. Reversal of chronic regional myocardial dysfunction (hibernating myocardium) by synchronized diastolic coronary venous retroperfusion during coronary angioplasty. J Am Coll Cardiol. 1990;15(1):238-242. 30. Kar S, Drury JK, Hajduczki I, et al. Synchronized coronary venous retroperfusion for support and salvage of ischemic myocardium during elective and failed angioplasty. J Am Coll Cardiol. 1991;18(1):271-282. C . 3 se 01 lU 2 na MP so H er ht r P rig Fo opy The Cardiac Dimensions Carillon system consists of a nitinol guide that has been molded to form a proximal and a distal anchor; these are joined by bridge elements with the ability to shorten the length of the coronary sinus.87 The first studies in humans found a decrease in mitral valve insufficiency in at least one degree in all cases, improving left ventricular function and decreasing ventricular diameter and volume. Likewise, a symptomatic improvement was achieved with a decrease in functional class and improvement after 6 minutes of continuous walking at 6-months follow-up exam.88 The Edwards Monarc system is made up of proximal and distal anchors formed by self-expanding stents, and a bridge system that joins them.89 The distal anchor is released in the anterior interventricular vein and the proximal anchor near the coronary sinus ostium. Once a large part of the mitral ring circumference is captured, the bridge segment is tensed in order to shorten it. The initial experience with this device showed a one or two degree reduction in insufficiency in most patients.90 However, the EVOLUTION II study (Clinical Evaluation of the Edwards Lifesciences Percutaneous Mitral Annuloplasty System for the Treatment of Mitral Regurgitation) was suspended due to patient inclusion difficulties. Lastly, the Viacor PTMA system91 consists of a trilumen catheter that may be implanted through the subclavian, with different nitinol guides that are advanced through the catheter lumen. Pressure is applied to the central portion of the posterior mitral ring, thus shortening the distance between the septum and the distal wall with a consequent shortening or constriction of the mitral ring.92 Initial studies showed a discrete decrease in the degree of insufficiency in most included patients, which was maintained throughout the first year of follow-up. However, the company suspended the research line and inclusion of new patients in the study.93 Although the devices are technically feasible to implant in most patients, technical problems have been described secondary to the anatomic variations of the coronary sinus, as well as material fatigue with device fracture, which has been described with the three devices and is related to the complex dynamics of compression and torsion that the device undergoes during the cardiac cycle. Another significant limitation is the compression of the circumflex artery by the device. 94 Most devices that cause compression of the circumflex artery present dramatic clinical manifestations during the procedure, forcing the removal of the device and cancellation of the procedure. Evaluation with multidetector scanning is very useful for detecting the anatomic relationship of the coronary sinus to the body of the circumflex artery and the marginal branches in order to decrease this complication. During the initial experience with these devices, their implantation was only successfully accomplished in two-thirds of the cases; most cases of failed implantation were due to anatomic variations of the sinus that make it unfavorable, cannulation difficulties with the guide catheter, or compression of the epicardial arteries. Even so, the results obtained at this time foreshadow a promising future for this type of device. 104 The Journal of Invasive Cardiology® Myocardial Venous Drainage: From Anatomy to Clinical Use enhances myocardial collateral perfusion in dogs. J Am Coll Cardiol. 2000;35(2):519-526. 66. von Degenfeld G, Raake P, Kupatt C, et al. Selective pressure-regulated retroinfusion of fibroblast growth factor-2 into the coronary vein enhances regional myocardial blood flow and function in pigs with chronic myocardial ischemia (see comment). J Am Coll Cardiol. 2003;42(6):1120-1128. 67. Menasche P, Hagege AA, Vilquin JT, et al. Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction (see comment). J Am Coll Cardiol. 2003;41:1078-1083. 68. Menasche P, Hagege AA, Scorsin M, et al. Myoblast transplantation for heart failure. Lancet. 2001;357(9252):279-280. 69. Reinecke H, MacDonald GH, Hauschka SD, Murry CE. Electromechanical coupling between skeletal and cardiac muscle. Implications for infarct repair. J Cell Biology. 2000;149:731-740. 70. Suzuki K, Murtuza B, Suzuki N, et al. Intracoronary infusion of skeletal myoblasts improves cardiac function in doxorubicin-induced heart failure. Circulation. 2001;104(12 Suppl 1):I213-I217. 71. Daubert JC, Ritter P, Le Breton H, et al. Permanent left ventricular pacing with transvenous leads inserted into the coronary veins. Pacing Clin Electrophysiol. 1998;21(1 Pt 2):239-245. 72. Stellbrink C, Diem B, Schauerte P, et al. Transcoronary venous radiofrequency catheter ablation of ventricular tachycardia. J Cardiovasc Electrophysiol. 1997;8(8):916-921. 73. Bristow MR, Saxon LA, Boehmer J, et al; Comparison of Medical Therapy, Pacing and Defibrillation in Heart Failure (COMPANION) Investigators. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350(21):2140-2150. 74. Cleland JG, Daubert JC, Erdmann E, et al; Cardiac Resynchronization-Heart Failure (CARE HF) Study Investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352(15):1539-1549. 75. Akiyama M, Kaneko Y, Taniguchi Y, et al. Coronary sinus recordings of double potentials associated with retrograde conduction through left atrioventricular accessory pathways. J Cardiovasc Electrophysiol. 2004;15(12):1371-1376. 76. Raatikainen MJ, Pedersen AK. Catheter ablation of a difficult accessory pathway guided by coronary sinus venography and 3D electroanatomical mapping. Europace. 2010;12(8):1200-1201. 77. Guzzo G, Cosío FG, Pastor A, Núñez A. Electroanatomic study of the left atrial insertion of an epicardial accessory pathway integrating the coronary sinus. Europace. 2010;12(7):1022-1024. 78. Aslani A, Moradi M, Kheirkhah J, Haghjoo M. Catheter ablation of an accessory pathway within the Marshall vein in a patient with mechanical mitral valve. Pacing Clin Electrophysiol. 2011 Mar 31. (Epub ahead of print). 79. Jimenez A, Shorofsky SR, Dickfeld TM, Anand R, Saliaris AP, Saba M. Left-sided atrial flutter originating in the coronary sinus after radiofrequency ablation of atrial fibrillation. Pacing Clin Electrophysiol. 2010;33(10):e96-e99. 80. Kanj M, Wazni O, Natale A. Pulmonary vein antrum isolation. Heart Rhythm. 2007;4(3):S73-S79. 81. Hwang C, Chen PS. Ligament of Marshall: why it is important for atrial fibrillation ablation. Heart Rhythm. 2009;6(12):S35-S40. 82. Sánchez-Quintana D, Ho SY, Cabrera JA, Farré J, Anderson RH. Topographic anatomy of the inferior pyramidal space: relevance to radiofrequency catheter ablation. J Cardiovasc Electrophysiol. 2001;12(2):210-217. 83. Reithmann C, Fiek M, Hahnefeld A, Ulbrich M, Steinbeck G. Recording of low-amplitude diastolic electrograms through the coronary veins: a guide for epicardial ventricular tachycardia ablation. Europace. 2011 Dec 23. (Epub ahead of print). 84. Jia-Feng L, Yue-Chun L, Jia L, et al. Successful epicardial ablation of idiopathic mitral annular ventricular tachycardia from the great cardiac vein. Pacing Clin Electrophysiol. 2011 Jan 5. (Epub ahead of print). 85. Yamada T, McElderry HT, Doppalapudi H, et al. Idiopathic ventricular arrhythmias originating from the left ventricular summit: anatomic concepts relevant to ablation. Circ Arrhythm Electrophysiol. 2010;3(6):616-623. 86. Byrne MJ, Kaye DM, Mathis M, et al. Percutaneous mitral anular reduction provides continued benefit in an ovine model of dilated cardiomyopathy. Circulation. 2004;110(19):3088-3092. 87. Maniu CV, Patel JB, Reuter DG, et al. Acute and chronic reduction of functional mitral regurgitation in experimental heart failure by percutaneous mitral annuloplasty. J Am Coll Cardiol. 2004;44(8):1652-1661. 88. Schofer J, Siminiak T, Haude M, et al. Percutaneous mitral annuloplasty for functional mitral regurgitation: results of the AMADEUS trial. Circulation. 2009;120(4):326-333. 89. Webb JG, Harnek J, Munt BI, et al. Percutaneous transvenous mitral annuloplasty: initial human experience with device implantation in the coronary sinus. Circulation. 2006;113(6):851-855. 90. Frerker C, Schäfer U, Schewel D, et al. Percutaneous approaches for mitral valve interventions — a real alternative technique for standard cardiac surgery? Herz. 2009;34(6):444-450. 91. Daimon M, Gillinov A, Liddicoat J, et al. Dynamic change in mitral annular area and motion during percutaneous mitral annuloplasty for ischemic mitral regurgitation: preliminary animal study with real-time 3-dimensional echocardiography. J Am Soc Echocardiogr. 2007;20(4):381-388. 92. Dubreuil O, Basmadjian A, Ducharme A, et al. Percutaneous mitral valve annuloplasty for ischemic mitral regurgitation: first in man experience with a temporary implant. Catheter Cardiovasc Interv. 2007;69(7):1053-1061. 93. Sack S, Kahlert P, Bilodeau L, et al. Percutaneous transvenous mitral annuloplasty: initial human experience with a novel coronary sinus implant device. Circ Cardiovasc Interv. 2009;2(4):277-284. 94. Tops L, Van de Veire N, Schuifj J, et al. Noninvasive evaluation of coronary sinus anatomy and its relation to the mitral valve annulus: implications for percutaneous mitral annuloplasty. Circulation. 2007;115(11):1426-1432. C . 3 se 01 lU 2 na MP so H er ht r P rig Fo opy 31. Beck CS, Stanton E, Batiuchok W, et al. Revascularisation of the heart by graft of systemic artery into coronary sinus. JAMA. 1948;137(5):436-442. 32. Beck CS, Leighninger DS. Operations for coronary artery disease. JAMA. 1954;156(13):1226-3123. 33. Oesterle SN, Reifart N, Hauptmann E, et al. Percutaneous in situ coronary venous arterialization. Report of the first human catheter-based coronary artery bypass. Circulation. 2001;103(21):2539-2543. 34. Boekstegers P, von Degenfeld G, Giehrl W, et al. Myocardial gene transfer by selective pressureregulated retroinfusion of coronary veins. Gene Therapy. 2000;7(3):232-240. 35. Hou D, Maclaughlin F, Thiesse M, et al. Widespread regional myocardial transfection by plasmid encoding Del-1 following retrograde coronary venous delivery. Catheter Cardiovasc Interv. 2003;58(2):207-211. 36. Losordo DW, Vale PR, Isner JM. Gene therapy for myocardial angiogenesis (review). Am Heart J. 1999;138(2 Pt 2):S132-S141. 37. Suzuki K, Murtuza B, Smolenski RT, Yacoub MH. Selective cell dissemination into the heart by retrograde intracoronary infusion in the rat. Transplantation. 2004;77(5):757-759. 38. Salukhe TV, Dimopoulos K, Francis D. Cardiac resynchronisation may reduce all-cause mortality: meta-analysis of preliminary COMPANION data with CONTAK-CD, InSync ICD, MIRACLE and MUSTIC. Int J Cardiol. 2004;93(2-3):101-103. 39. Cappato R, Schluter M, Weiss C, et al. Mapping of the coronary sinus and great cardiac vein using a 2-French electrode catheter and a right femoral approach. J Cardiovasc Electrophysiol. 1997;8(4):371-376. 40. Linde C, Braunschweig F, Gadler F, Bailleul C, Daubert JC. Long-term improvements in quality of life by biventricular pacing in patients with chronic heart failure: results from the Multisite Stimulation in Cardiomyopathy study (MUSTIC). Am J Cardiol. 2003;91(9):1090-1095. 41. Chatterjee K, Magnusson P, Kaushik VS, Swan HJ. Selective cannulation of the anterior interventricular vein and coronary sinus: potential applications. Cathet Cardiovasc Diagn. 1977;3(1):91-99. 42. Katritsis D, Webb-Peploe MM. Cannulation of the coronary sinus via the femoral vein — a new technique. Clin Cardiol. 1997;20(5):446-448. 43. Sayad DE, Sawar A, Curkovic V, et al. Simple access to the coronary venous system for left ventricular pacing. Pacing Clin Electrophys. 2003;26(9):1856-1858 44. Langenberg CJ, Pietersen HG, Geskes G, et al. Coronary sinus catheter placement: assessment of placement criteria and cardiac complications. Chest. 2003;124(4):1259-1265. 45. Pratt FH. The nutrition of the heart through the vessels of Thebesius and the coronary veins. Am J Physiol. 1898;1:86. 46. Batson OV, Bellet S. The reversal of flow in the cardiac veins. Am Heart J. 1930;6:206. 47. Gross L, Blum L, Silverman G. Experimental attempts to increase blood supply to the dogs heart by means of coronary sinus occlusion. J Exp Med. 1937;65(1):91. 48. Arealis EG, Volder JG, Kolff WJ. Arterialization of the coronary vein coming from an ischemic area. Chest. 1973;63(3):462-463. 49. Benedict JS, Buhl TL, Henney RP. Cardiac vein myocardial revascularisation, an experimental study and report of three clinical cases. Ann Thorac Surg. 1975;20(5):550-557. 50. Park SB, Magovern GJ, Magovern GJ. Direct selective myocardial revascularisation by internalmammary artery-coronary vein anastamosis. J Thorac Cardiovasc Surg. 1975;69(1):63-72. 51. Gore JM, Weiner BH, Benotti JR, et al. Preliminary experience with synchronized coronary sinus retroperfusion in humans. Circulation. 1986;74(2):381-388. 52. Kar S, Drury JK, Hajduczki I, et al. Synchronized coronary venous retroperfusion for support and salvage of ischemic myocardium during elective and failed angioplasty. J Am Coll Cardiol. 1991;18(1):271-282. 53. Berland J, Farcot JC, Barrier A, et al. Coronary venous synchronized retroperfusion during percutaneous transluminal angioplasty of left anterior descending coronary artery. Circulation. 1990;81(3 Suppl):IV35-IV42. 54. Oesterle SN, Reifart N, Hayase M, et al. Catheter-based coronary bypass: a development update. Catheter Cardiovasc Interv. 2003;58(2):212-218. 55. Jain AK, Mathur A, Rothman MT. The marginal coronary vein is a target for percutaneous retrograde coronary venous perfusion. TCT 2004. Presented at the Transcatheter Cardiovascular Therapeutics meeting, Washington D.C. 56. Yoneyama R, Kawase Y, Hoshino K, et al. Magnetic resonance assessment of myocardial perfusion via catheter-based ventricle-coronary vein bypass in porcine myocardial infarction model. Catheter Cardiovasc Interv. 2006;67(1):58-67. 57. Haga Y, Uriuda Y, Bjorkman JA, et al. Ischemic and nonischemic tissue concentrations of felodipine after coronary venous retroinfusion during myocardial ischemia and reperfusion: an experimental study in pigs. J Cardiovasc Pharm. 1994;24(2):298-302. 58. Hatori N, Sjoquist PO, Regardh C, Ryden L. Pharmacokinetic analysis of coronary sinus retroinfusion in pigs. Ischemic myocardial concentrations in the left circumflex coronary arterial area using metoprolol as a tracer. Cardiovasc Drug Ther. 1991;5(6):1005-1010. 59. Ryden L, Tadokoro H, Sjoquist PO, et al. Pharmacokinetic analysis of coronary venous retroinfusion: a comparison with anterograde coronary artery drug administration using metoprolol as a tracer. J Am Coll Cardiol. 1991;18(2):603-612. 60. Ryden L, Tadokoro H, Sjoquist PO, et al. Pronounced accumulation of metoprolol in ischemic myocardium after coronary venous retroinfusion. J Cardiovasc Pharmacol. 1990;15(1):22-28. 61. Raake P, von Degenfeld G, Hinkel R, et al. Myocardial gene transfer by selective pessure-regulated retroinfusion of coronary veins: comparison with surgical and percutaneous intramyocardial gene delivery. J Am Coll Cardiol. 2004;44(5):1124-1129. 62. Penta K, Varner JA, Liaw L, et al. Del1 induces integrin signaling and angiogenesis by ligation of alphaVbeta3. J Biological Chem. 1999;274(16):11101-11109. 63. Schumacher B, Pecher P, von Specht BU, Stegmann T. Induction of neoangiogenesis in ischemic myocardium by human growth factors: first clinical results of a new treatment of coronary heart disease (see comment). Circulation. 1998;97(7):645-650. 64. Sato K, Wu T, Laham RJ, et al. Efficacy of intracoronary or intravenous VEGF165 in a pig model of chronic myocardial ischemia. J Am Coll Cardiol. 2001;37(2):616-623. 65. Rajanayagam MA, Shou M, Thirumurti V, et al. Intracoronary basic fibroblast growth factor Vol. 25, No. 2, February 2013 105