* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Sec. 10.3 - Midland Park School District
Chemistry: A Volatile History wikipedia , lookup
Stöber process wikipedia , lookup
Fluorescence correlation spectroscopy wikipedia , lookup
Brownian motion wikipedia , lookup
Elastic recoil detection wikipedia , lookup
History of chemistry wikipedia , lookup
Chemical bond wikipedia , lookup
Depletion force wikipedia , lookup
Debye–Hückel equation wikipedia , lookup
Inductively coupled plasma mass spectrometry wikipedia , lookup
Molecular dynamics wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Metalloprotein wikipedia , lookup
Elementary particle wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
Size-exclusion chromatography wikipedia , lookup
Particle-size distribution wikipedia , lookup
Stoichiometry wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
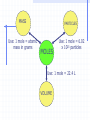
Ch. 10: The Mole *Part of Sec. 13.2: Avogadro’s Principle (pages 452-453) Sec. 10.3: Moles of Compounds (pages 333 – 334 & 338 - 339) Moles of Compounds Avogadro’s Principle Relate numbers of particles and volumes by using Avogadro’s principle. Recognize the mole relationships shown by a chemical formula. Determine the number of atoms or ions in a mass of a compound. Avogadros’ Principle O O C O The particles making up different gases can vary greatly in size. O Avogadros’ Principle Yet, the particles in all gases are far enough away from each other that their size has no effect on the amount of space (volume) a sample occupies. All gases are mostly empty space. Avogadros’ Principle That means, at the same temperature and pressure, 1000 oxygen molecules will occupy the same space as 1000 carbon dioxide molecules. They both occupy the same space as 1000 helium atoms. This is Avogadro’s Principle. Avogadros’ Principle Avogadro’s Principle states that equal volumes of gases at the same temperature and pressure contain equal numbers of particles. Further, one mole (or 6.02 x 1023 particles) of a gas (no matter what it is) will always have the same volume. Molar Volume The molar volume of a gas is the volume occupied by one mole of a gas at STP. STP stands for standard temperature and pressure: 0o C and 1 atm. pressure. Avogadro showed that 1 mole of any gas will occupy a volume of 22.4 liters at STP. Molar Volume OR 1 mole of any gas will occupy 22.4 L of space. Mole/Volume Conversions Calculate the volume that 0.881 mol of helium will occupy at STP. 0.881 mol x 22.4 L = 19.7 L 1 mole Determine the volume of a container that holds 2.4 mol of gas at STP. How many moles of nitrogen gas will be contained in a 2 L flask at STP? Molar Volume Now it is possible to relate moles, mass, # of particles and volume for all gases. 1 mole = 6.02 x 1023 particles = atomic or formula mass in grams = 22.4 L MASS Use: 1 mole = atomic mass in grams PARTICLES MOLES Use: 1 mole = 6.02 x 1023 particles Use: 1 mole = 22.4 L VOLUME Mass/Volume Conversions Calculate the volume that 50 g of methane (CH4) will occupy at STP. 50 g x 1 mole = 3.1 mol x 22.4 L = 69 L 16 g 1 mol What volume will 0.416 g of krypton gas occupy at STP? How many grams of carbon dioxide gas are in a 1.0 L balloon? Chemical Formulas & the Mole The formula CCl2F2 (freon) indicates that 1 molecule of CCl2F2 contains 1 C atom, 2 Cl atoms, and 2 F atoms. What if we multiply all the numbers in this expression by 6.02 x 1023?*** This means, 1 mole of CCl2F2 will contain 1 mole of C atoms, 2 moles of Cl atoms, and 2 moles of F atoms. These equivalencies that we find in the formula can be written as conversion factors. They are . . How many moles of fluorine atoms are in 5.5 moles of freon? Chemical Formulas & the Mole 5.5 moles CCl2F2 x 2 moles F atoms 1 mole CCl2F2 = 11.0 moles F atoms Chemical Formulas & the Mole Conversion factors can be determined for any element or ion from the formula of a compound. In the conversion factor, the subscript for an element or ion in the formula is the number of moles of that element or ion in one mole of the compound. Practice Problems 1. 1 mole Al2(SO4)3 contains: _____ mol Al+3 ions _____ mol SO4-2 ions _____ mol O atoms 2. 4.5 mol Ca3(PO4)2 contains: _____ mol Ca+2 ions _____ mol PO4-3 ions _____ mol O atoms More Practice Problems Determine the moles of aluminum ions (Al+3) in 1.25 moles of aluminum oxide (Al2O3). How many moles of oxygen atoms are present in 5.0 mol diphosphorus pentoxide (P2O5)? Determine the number of moles of oxygen atoms in 0.34 mol of oxygen gas (O2). Converting Mass to # of Particles Remember: # of Particles Mass Converting Mass to # of Particles Now, in addition to the conversions pictured, you can determine the # of atoms or ions in a compound by using a conversion factor that is written from the formula of a compound. Converting Mass to # of Particles A sample of silver chromate (Ag2CrO4) has a mass of 25.8 g. How many Ag+ ions are present? Step 1: mass given to moles compound 25.8 g Ag2CrO4 x 1 mole = 0.0778 mol Ag2CrO4 331.8 g Converting Mass to # of Particles Step 2: moles compound to moles particles 0.0778 mol Ag2CrO4 x 2 moles Ag+ = 0.156 moles Ag+ 1 mol Ag2CrO4 Step 3: moles particles to # of particles 0.156 moles Ag+ x 6.02 x 1023 Ag+ ions 1 mole Ag+ = 9.39 x 1022 Ag+ ions Practice Problems A sample of aluminum chloride has a mass of 35.6 g. How many Cl- ions are present in the sample? How many moles of calcium ions are present in 1 g of CaCO3? How many calcium ions are present? What mass of sodium chloride (NaCl) contains 4.59 x 1024 Cl- ions? A sample of Pb(NO3)2 has 8.2 x 1025 formula units. How many nitrate ions does it contain?