* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Mass - Mass Relationships
Survey
Document related concepts
Nucleophilic acyl substitution wikipedia , lookup
Sodium hydroxide wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Silver as an investment wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
Transcript
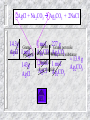
Notes #3 Grams to grams stoichiometry 4- step bridge MASS-MASS STOICHIOMETRY!! Example: How many grams of silver chloride are produced when 17.0 grams of silver (I) nitrate react with excess sodium chloride? Mass - Mass Relationships Write a balanced equation for the reaction. (Remember to look up the oxidation numbers so the formulas are correct). Start with mass given. Calculate the number of moles of the “given” substance. AgNO3 = 170g/mole(molar mass conversion factor) Use the mole ratio to determine the moles of the “required” (unknown) substance to moles of the “given” substance.(Use the balanced equation.) Convert moles “required” to grams(molar mass conversion factor) Mass - Mass Relationships AgNO3 + NaCl AgCl + NaNO3 given required Grams of Start w/ 1 mole of Moles grams given given “required” “required” Grams of given 17.0 g AgNO3 1 Mole AgNO3 169.88g AgNO3 Moles given 1 mole “required” 1 mol AgCl 1 mol AgNO3 = grams produced 143.32 gAgCl = 14.3 g AgCl 1 mol AgCl Problems: How much silver carbonate is produced when 14.3 g of silver chloride reacts with excess sodium carbonate? What kind of reaction is this? Double displacement. Write the balanced equation. 2 AgCl + Na2CO3 Ag2CO3 + 2NaCl 2AgCl + Na2CO3 Ag2CO3 + 2NaCl 14.3g 1 mol 277 g Moles of Grams Grams per mole mol Given 1 per AgCl Ag CO Ag CO silver carbonate mole of required 2 3 2 3 substance AgCl = 13.9 g Moles of 1 mol 2 mol 143g Ag2CO3 silver chloride AgCl Ag2CO3 AgCl Practice problems 1. In a spacecraft, the carbon dioxide exhaled by astronauts can be removed by its reaction with lithium hydroxide according to the following equation: CO2 (g) + 2LiOH Li2CO3(s) + H2O (l) How many moles of lithium hydroxide are required to react with 20 moles of caron dioxide?