* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Raman spectroscopy
Spin (physics) wikipedia , lookup
Molecular Hamiltonian wikipedia , lookup
Renormalization wikipedia , lookup
Ferromagnetism wikipedia , lookup
Particle in a box wikipedia , lookup
Wave–particle duality wikipedia , lookup
Molecular orbital wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Symmetry in quantum mechanics wikipedia , lookup
Tight binding wikipedia , lookup
Mössbauer spectroscopy wikipedia , lookup
Rotational–vibrational spectroscopy wikipedia , lookup
Rotational spectroscopy wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Franck–Condon principle wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Atomic theory wikipedia , lookup
Atomic orbital wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
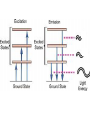
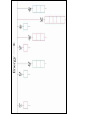
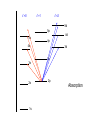
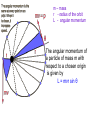
Raman spectroscopy • You are already aware that photons interact with molecules to induce transitions between energy states. • In Raman spectroscopy- a photon is scattered by the molecular system. Raleigh Scatter (same wavelength as incident light) Raman Scatter (new wavelength) • Elastic, or Raleigh scattering : the photon simply 'bounces' off the molecule, with no exchange in energy. • Inelastic, or Raman scattering: there is an exchange of energy between the photon and the molecule, leading to the emission of another photon with a different frequency to the incident photon 2 • Raman scattering can occur with a change in vibrational, rotational or electronic energy of a molecule. • Chemists are concerned primarily with the vibrational Raman effect. The Raman effect arises when a photon is incident on a molecule and interacts with the electric dipole of the molecule. This interaction can be viewed as a perturbation of the molecule’s electric field - Energy level diagram for Raman scattering - scattered photon have (a) lower energy (b) higher energy than the exciting photon The different possibilities of visual light scattering: Rayleigh scattering Stokes scattering (molecule absorbs energy) and anti-Stokes scattering (molecule loses energy) • For Raman scattering to occur, the polarizability of the molecule must vary with its orientation. • One of the strengths of Raman spectroscopy is that this will be true for both heteronuclear and homonuclear diatomic molecules. • Homonuclear diatomic molecules do not possess a permanent electric dipole, and so are undetectable by other methods such as infrared. • The polarizabilities of these two homonuclear diatomic molecules will be different. • Polarizability is the relative tendency of the electron cloud of an atom to be distorted from its normal shape by the presence of a nearby ion or dipole : that is, by an external electric field EXAMPLE: RAMAN SPECTRUM OF CCl4 COMPARISON BETWEEN IR AND RAMAN SPECTROSCOPIES IR Absorption RAMAN Scattering Requires change in Dipole moment Requires change in polarizability. Aqueous samples difficult To examine Aqueous samples readily examined. Glass or quartz unsuitable As IR cells. NaCl, KBr, CaF2 are suitable. Glass or quartz suitable Electronic spectroscopy Electronic energy levels of a molecule gives much more information about the structure of molecules • Atomic spectrum • • • - consists of series of lines or sharply defined emission or adsorption peaks • • • • - because, in the vapour phase they can neither vibrate or rotate and have few collisions with each other V or λ • Electrons can get into the excited states by either colliding with other atoms or absorbing photons of specific energies. • Absorption Lines: • - Can be observed when an electron absorbs a photon with exactly the energy needed to jump from a lower to a higher orbital. Absorption is very specific:Only photons with the exact excitation energy are absorbed. - All others pass through unabsorbed. A continuous spectrum from the lamp crossed by of dark "absorption lines" at particular wavelengths • Emission Lines: • When an electron jumps from a higher to a lower energy orbital, a single photon is emitted with exactly the energy difference between orbitals. No more, no less. • Emits only at particular wavelengths, giving the appearance of bright, discrete "emission lines" Darkness in between the emission lines • Comparison of the emission and absorption lines of sodium • Each element have a characteristic line spectrum. • - It is a reflection of the detailed structure of the atom. Depends on the number and arrangement of electrons in orbit around the nucleus. • The Spectral Lines are a kind of "signature" for the atoms. • The spectra of molecules are quite different from those of the atom Hydrogen spectra from (a) molecular hydrogen (H2) and (b) atomic hydrogen • • • • • Atomic orbital and their energies An atomic orbital is a one electron wave function Each hydrogenic atom is defined by 3 quantum numbers Principle quantum number n = 1, 2, 3,………… Angular momentum quantum number l = 0, 1, 2, 3, ………… Magnetic quantum number m l = 0, ±1, ± 2, ± 3, ………… • A electron in an orbital with quantum number n has an energy me e 4 hcRH En 2 2 2 2 n 8 0 h n • • • • • • Me- mass of an electron E - charge of an electron ε0- vacuum permittivity n – principal quantum number h – Plank’s constant RH – Rydberg constant • Principal quantum number • n 1 2 3 4……….. • K L M N ………… • Sub cells are given by • • l =0 1 2 3 4 s p d f g 5 6 ………… h i …….. • The orbitals with same value of n, but different values of l are said to form a sub-shell of a given shell • Hydrogen: The Simplest Atom An atom of Hydrogen (1H) consists of: - A single proton in the nucleus. - A single electron orbiting the nucleus. First orbital: Ground State (n=1) Lowest energy orbital the electron can reside in. Higher orbitals: Excited States (n=2,3,...) - Higher orbits around the nucleus. - Come at specific, exact energies. • Selection rules which govern the transition of electron between energy levels of hydrogenic atoms (He+, Li2+ , Be3+ and , B4+ ) • 1. Δn any value • 2. An s electron can not make any transition to another s orbital • 3. Electron in a d orbital (l =2) can not make a transition to an s • orbital (l =0) • Therefore the derived selection rules for hydrogenic atoms are: • Δn any value , s s • Δl = ±1 • eg. l =1 (p) • l =2 (d) l =0 (s) • Therefore an electron in the ground state (1s) can undergo any transition to any p state • 1s np (n≥ 2) • 2p ns or nd l =0 l =1 l =2 5d 5p 5s 4d 4p 4s 3d 3p 3s 2s Forbidden 2p Allowed transition transition 1s Absorption l =0 l =1 l =2 5d 5p 5s 4d 4p 4s 3d 3p 3s 2s 1s 2p Absorption l =0 l =1 l =3 l =2 5f 5d 4f 5p 5s 4d 4p 4s 3d 3p 3s 2s 1s 2p Absorption l =0 l =1 Paschen series l =2 5d 5p 4d 5s 4p 4s 3d 3p 3s Balmer series 2p 2s 1s Emission Lyman series How other quantum numbers beside n affect the electronic energy levels? • Orbital angular momentum(l ) • Electrons are rotating around its nucleus creating orbital angular momentum • This momentum is quantized h l l (l 1) . 2 • l – is the orbital angular momentum quantum number l = 0, 1, 2, 3, ………… m – mass r - radius of the orbit L - angular momentum The angular momentum of a particle of mass m with respect to a chosen origin is given by L = mvr sin θ • Electron spin angular momentum (S) • Every electron in an atom can spin about its own axis as well as rotate around the nucleolus. • The spin motion can be expressed by the following expression h s s ( s 1) . 2 • • • s– is the spin angular momentum quantum number and it is a half-integral for a one electron atom (±1/2) • Total electronic angular momentum j l s • Spin orbital coupling • An electron has a spin angular momentum and it also has a magnetic momentum that arises from its spin • ( a moving charge generates a magnetic field) • Electron with orbital angular momentum creates a circulating current and possesses a magnetic moment that arises form its orbital momentum • The interaction of spin & orbital magnetic moments is called spin-orbital coupling • Energy levels are split as a result of spin-orbital coupling • (j –splitting) • Total electronic angular momentum j l s • When l =1 (p electron) s = ±1/2 • • • • • - therefore j = 3 ∕ 2 or 1 ∕ 2 - the p state is split into two energy states l =2 (d electron) s = ±1/2 - therefore j = 5 ∕ 2 or 3 ∕ 2 - the d state is split into two energy states Energy 3d 3p 3/2 1/2 3s 2p 2s 1s 1/2 5/2 3/2 3/2 1/2 * s = -1/2 is not allowed, since a quantum number can not be negative • Selection rules • Δn any value • Δ l = ±1 • Δ j = 0, ±1 • Therefore allowed transition between s and p levels are • s1/2 p1/2 (Δ j = 0) • s1/2 p3/2 (Δ j = +1) • Every line in the H-spectrum is split into two as a result of • j-splitting • -gives doublets • -the separation between lines are too small to be • resolved • The inclusion of coupling between orbitals and spin momentum has sligtly increased the complexicity of H-spectrum • The selection rules for the splitting for alkali metals (Li, Na, K, Rb, Cs) are the same as hydrogen • Δn any value • Δ l = ±1 • Δ j = 0, ±1 • The spiting due to l and s incerases remarkably with the atomic number • H – can not separate • Li – less than 1 cm-1 • Na – 17 cm-1 • Cs – 500 cm-1