* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Antibacterials II: Vancomycin, Linezolid, Daptomycin, Macrolides
Pharmaceutical industry wikipedia , lookup
Pharmacognosy wikipedia , lookup
Prescription costs wikipedia , lookup
Drug design wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Levofloxacin wikipedia , lookup
Drug discovery wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Drug interaction wikipedia , lookup
Neuropharmacology wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Discovery and development of cephalosporins wikipedia , lookup
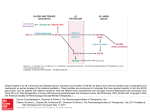
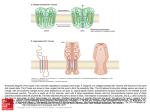
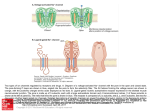
Vancomycin Class: Glycopeptide antibiotic MOA: Inhibition of bacterial cell wall synthesis by binding Dala-D-ala Goodman & Gilman’s The Pharmacological Basis of Therapeutics – 11th Ed. (2006) Peptidoglycan Synthesis “Penicillin binding protein” Vancomycin IV, PO Spectrum: Gram (+) Drug of Choice MRSA Indications IV: Serious methicillin-resistant Staphylococcal infections: pneumonia, endocarditis, osteomyelitis, SSSI PO: pseudomembranous colitis (metronidazole preferred) Staphylococcal infections in Penicillin allergic patients NOTE: Do not use in non-Penicillin allergic patients. Vancomycin does not kill as rapidly as antistaphylococcal β-lactams, and may negatively impact clinical outcome Unique Qualities Monitor trough serum concentrations Poor oral absorption Adjust dose for renal impairment ADRs “Red Man” Syndrome Ototoxicity Nephrotoxicity w/ other nephrotoxic agents Vancomycin Mechanism of action: Inhibits bacterial cell wall synthesis Spectrum of action: Gram positive organisms Including: Listeria, Rhodococcus, Peptostreptococcus Bacteriostatic against enterococcus Mechanism of resistance: Enterococcus: Van A – E Peptidoglycan precursor has decreased affinity for vancomycin – D-ala-D-ala replaced by D-ala-D-lac Staphylococcus aureus: VISA isolates: • Increased amount of precursor with decreased affinity • Thicker cell wall hVISA: heterogenous bacterial population Vancomycin Dose: Based on total body weight and renal function 15 – 20 mg/kg Normal renal function: q 12 dosing Goal trough concentrations: 10 – 15 mcg/mL: bacteremia, skin and soft tissue infections 15 – 20 mcg/mL: osteomyelitis, meningitis, pneumonia Linezolid Class: Oxazolidinedione MOA: Binds P site of 50s ribosomal subunit, preventing translation initiation Goodman & Gilman’s The Pharmacological Basis of Therapeutics – 11th Ed. (2006) http://www.chm.bris.ac.uk/motm/linezolid/linezolid.htm Linezolid IV, PO Gram (+) Indications VRE (E. faecium) Nosocomial pneumonia (S. aureus) Community-acquired pneumonia (S. pneumoniae) cSSSI (S. aureus) Unique Qualities F~100%, IV=PO Reserve use for treatment of multiple drug resistant strains No CYP interaction ADRs Generally well tolerated w/ minor SE in short term Rx Myelosuppression: anemia, leukopenia, pancytopenia, thrombocytopenia Peripheral and optic neuropathy Linezolid Penetration: Plasma Pulmonary lining Blister fluid > MIC90 for Staphylococcus Dose (IV or PO): 600 mg Q12H Drug-drug interactions: Non-selective inhibitor of MAO Possible serotonergic or adrenergic interaction with antidepressant medications (incidence < 1%) Daptomycin Class: Cyclic lipopeptide MOA: In the presence of Ca2+, binds bacterial membrane resulting in depolarization Goodman & Gilman’s The Pharmacological Basis of Therapeutics – 11th Ed. (2006) http://cubicin.com/am_moa.htm Daptomycin Indications: Treatment of complicated SSTI’s caused by gram positive bacteria Treatment of Staphylococcus bacteremia and right-sided endocarditis Not used for treatment of pneumonia due to binding reaction with surfactant inactivates daptomycin MOA: Binds membrane Rapid depolarization Cell death Daptomycin Pharmacokinetic profile: Concentration-dependent killing Post-antibiotic effect Available for intravenous use only Penetration: Good penetration into vascular tissues and plasma Currently testing penetration into cerebral spinal fluid Dose: SSTIs: 4 mg/kg IV daily Bacteremia: 6 mg/kg IV daily Adjust for decreased renal function – CrCl < 30, use qod Interacts with assay for INR testing – results in falsely high INR recommend point of care testing Macrolide Mechanism of Action Bacteriostatic Inhibits protein synthesis Bind reversibly to 50s unit of the ribosome Blocks translocation of peptides from A-site to P-site. Goodman and Gilman’s The Pharmacological Basic of Therapeutics. 11ed. 2006 Macrolides Achieve higher tissue than plasma concentrations Penetrate into respiratory, tonsillar, and prostate tissues Also penetrate into PMN leukocytes Important for Atypicals like: Chlamydia and Legionella species PD: Time the bacteria is exposed to therapeutic concentrations above the MIC best predicts efficacy – time dependent killing Clarithromycin 14-membered lactone ring Replace hydroxyl group at C-6 position with methoxyl group Increase stability under acidic conditions Partially metabolized via CPYP3A4 converted to active metabolite 14-OHclarithromycin Primarily excreted in urine Goodman and Gilman’s The Pharmacological Basic of Therapeutics. 11ed. 2006 Clarithromycin PO: Biaxin® 250-500 mg q 12 hours; Biaxin XL® 1000 mg qday Spectrum of Activity: Gram (+) and Gram (-) Indications: otitis media, CAP, pharyngitis/tonsillitis, sinusitis, uncomplicated skin infections, prevention of MAC, duodenal ulcer disease S. aures, S. pyogenes, S. pneumoniae, Mycobacterium avium complex C. pneumoniae, C. trachomatis, L. pneumoniae H. influenzae, H.pylori Drug Interactions: Substrate of CYP 3A4 and Inhibits CPY 3A4(major) CYP 1A2 (weak) Theophylline, statins, digoxin, warfarin, cyclosporine Renal Adjustments: CrCl < 30 ml/min: ½ the normal dose or double the dosing interval ADR: Prolongs the QT interval – use with caution in CAD N/V, diarrhea, headache Counseling Points: Take XL formulation with food; do not chew or crush Azithromycin 15-membered lactone ring N-methyl group inserted between C-9 and C-10 Ketone replaced with – CH2 Goodman and Gilman’s The Pharmacological Basic of Therapeutics. 11ed. 2006 Azithromycin PO, IV Azithromyicn: 500 mg x day 1 then 250 mg x day 2-5 STDs: C. trachomatis: 1 g x 1; N. gonorrheae: 2 g x 1 Spectrum of Activity: Less Gram (+), increased Gram (-) Indications: otitis media, pharyngitis/tonsillitis, upper and lower respiratory tract infections, skin and skin structure, CAP, PID, STDs S. aures, S. pneumoniae, H. influenzae, Mycobacterium avium complex C. trachomatis, M. catarrhalis, M. pneumonia, N. gonorrheae, Chlamydia pneumoniae Drug Interactions: not as significant as other macrolides Most documented with cyclosporine and tacrolimus Unique Characteristics: T ½ of immediate release: 68-72 hours; extended release: 59 hours Caution in patients with CrCl < 10 ml/min ADRs: Generally well-tolerated, may cause GI upset Macrolide Resistance Decrease of permeation of drug through the cell membrane, or drug efflux pumps Methylase modifies the ribosomal target Hydrolysis of macrolides by endogenous esterase Telithromycin: Ketolide 1st of ketolide class Designed to target macrolide-resistant respiratory tract pathogens Compared to macrolidemore highly concentrated in tissue Not currently recommended in guidelines due to hepatotoxicity Goodman and Gilman’s The Pharmacological Basic of Therapeutics. 11ed. 2006 Telithromycin (Ketek ®) PO: CAP-800 mg qday x 7-10 days Spectrum of activity: Gram (+) and Gram (-) Indications: acute exacerbations of chronic bronchitis, acute sinusitis, CAP Staphylococci, S. pneumoniae (DRSP), H. influenzae, Moraxella catarrhalis, mycoplasma, chlamydia, Legionella Drug Interactions: Inhibits CYP2D69(weak) 3A4(strong): Multiple Drug Interactions ADRs Hepatotoxicity: Monitor LFTs, sxs of liver failure QT prolongation N/V: take with or without food Dose adjust for renal insufficiency Goodman and Gilman’s The Pharmacological Basic of Therapeutics. 11ed. 2006 Clindamycin Class: Lincosamide Mechanism of Action: Binds exclusively to the 50S subunit of bacterial ribosomes and suppress protein synthesis Clindamycin Trade names: Cleocin ®, Clindesse®, Clindagel ®, Clindamax ®, Evoclin ® Delivery forms: capsules: 75, 150, 300 mg; granules for oral solution 75mg/5ml; injection 150 mg/ml; vaginal cream 2%; vaginal suppositories 100 mg; topical gel 1%; topical lotion 1%; topical solution 1%; foam 1% Clindamycin Indications: Serious infections caused by susceptible anaerobic bacteria Dosing: Adults:150-450 mg Q 6 hrs Children:8-20 mg/kg/day divided TID-QID Off-label indications: CNS toxoplasmosis in AIDS patients in addition to pyrimethamine; chlamydia infections in women; bacterial vaginosis due to Gardnerella vaginalis Instructions: Take with full glass of water Warning: Pseudomembranous colitis Clindamycin Precautions: Renal impairment/liver disease Elderly Meningitis GI disease Superinfections Pregnancy Category B Drug Interactions: Erythromycin Neuromuscular blocking agents ADRs: Dermatologic, GI, Hypersenstivity Aminoglycosides • Bactericidal inhibitors of protein synthesis • Concentration dependent bacteria killing • Postantibiotic effect • Major limitation of use is the serious toxicity • Nephrotoxicity • Ototoxicity Aminoglycosides: Indications • Primarily against aerobic, gram negative bacilli • Activity against gram positive bacteria limited • Synergistic effect against “sensitive” (highlevel) streptococci and enterococci when used with a cell wall active agent Penetrating the Cell Gram Negative Bacteria • Diffuse through porin proteins on the outer membrane of gram negative cell wall • Transport across inner membrane depends on electron transport • Membrane potential drives permeation • Transport can be blocked by reduction in pH and anaerobic environment Adapted from: http://web.indstate.edu/thcme/micro/respiratory/sld006.htm Mechanism of Action • Bactericidal • Inhibit protein synthesis • Bind to bacterial 30S ribosomal subunit Blocks initiation of protein synthesis Cause misreading of mRNA template Cause premature termination of translocation Goodman and Gilman’s Aminoglycosides: Resistance Modes of resistance • Decreased permeation of aminoglycosides • Low affinity for bacterial ribosome • Drug inactivation by microbial enzymes • Important clinically • Amikacin is less vulnerable Structure Goodman and Gilman’s Aminoglycosides Resistance: Intrinsic vs. Acquired Intrinsic: Anaerobes: lack active electron transport chain to cross membrane Mutation at 16s rRNA (ie TB) Acquired: Efflux: seen in Pseudomonas Decreased transmembrane potential: seen in Enterococcus Aminoglycosides Distribution: Freely into the vascular space Interstitial spaces of most tissues Volume of distribution increases in edematous states and decreases in obese patients (on L/kg basis) Decreased concentrations: Bronchial secretions, CSF, biliary tract, synovial fluid, and in the eye Excreted by the kidneys Half-life: 1.5 to 3.5 hours Aminoglycosides Toxicity: Nephrotoxicity: Incidence 5% to 25% Risk factors: Renal Disease Hypotension Hepatic dysfunction Frequent dosing interval Larger doses Other nephrotoxic medications Increased Age Treatment > 3 days Ototoxicity (cochlear, vestibular) Neuromuscular blockade (very rare) Toxicity • Dependent on: • Total amount of drug AND duration of therapy • Nephrotoxicity • • • • Most often reversible Accumulation of drug in proximal tubular cells Mild rise in Scr (0.5-1 mg/dl) Reduced excretion of drug = increased risk of ototoxicity • Ototoxicity • Largely irreversible if not caught early • Destruction of vestibular and cochlear sensory cells • High-pitched tinnitus is often 1st symptom Aminoglycosides Site of infection: determines goal levels and dose Peak concentrations: Gram + Synergy: 3 – 5 mcg/mL UTI: 3 – 4 mcg/mL Bacteremia: 6 – 8 mcg/mL Pneumonia: 8 – 10 mcg/mL Weight based dosing: use IBW or ABW Interval: once-daily dosing for gram-negative infection (normal renal function, 7 mg/kg/day). Gram + synergy 1mg/kg q 8-12h. Gentamicin: Once Daily Dosing • 5-7mg/kg/24hrs (ABW) • Target peak 14-20 mcg/ml • Allows low troughs • Avoid in patients with: • Burns, CF, pregnancy, children, endocarditis or CrCl < 20ml/min Tobramycin • Antimicrobial activity and PK properties very similar to gentamicin • Superior activity against P. aeruginosa • Less active than gentamicin against enterococci • Can be given IV or IM • Dosage and serum levels are same as gentamicin Amikacin • Broadest spectrum of activity • Resistant to aminoglycoside-inactivating enzymes • Less active against enterococci • Similar dosing interval and monitoring • Peak • Life-threatening infection • Serious infection 25-30 mcg/ml 20-25 mcg/ml • Trough • Life threatening infection • Most infections 4-8 mcg/ml 1-4 mcg/ml