* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 06-zonosis_2
Avian influenza wikipedia , lookup
Foot-and-mouth disease wikipedia , lookup
Human cytomegalovirus wikipedia , lookup
Taura syndrome wikipedia , lookup
Hepatitis C wikipedia , lookup
Influenza A virus wikipedia , lookup
Hepatitis B wikipedia , lookup
Canine distemper wikipedia , lookup
Canine parvovirus wikipedia , lookup
Orthohantavirus wikipedia , lookup
Marburg virus disease wikipedia , lookup
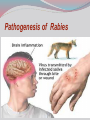
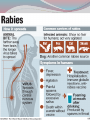
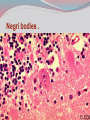
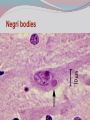
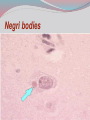
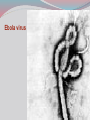
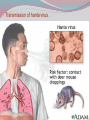
Dr: MONA BADR Assistant professor Consultant virologist Viral zoonotic diseases Divided into two groups: 1- Arboviruses zoonotic diseases. They are transmitted from animal to human through the bite of arthropode sucking blood vector such as mosquitoes, ticks and sand flies . Arboviruses cause hemorrhagic fever , encephalitis or febrile illness . Viral zoonotic diseases 2- Non arboviruses zoonotic diseases. This group include rabies, Marburg, Ebola , Lassa and Hanta viruses .They are transmitted from infected animal to human through the bite of rabid animal (rabies) , direct exposure to blood and tissues from infected monkeys , ( Marburg & Ebola ) or direct exposure to infected rodents excreta ( Lassa and hanta). RABIES Rabies is also known as hydrophobia. Rabies virus causes an encephalomyelitis leading to degeneration of neurons in CNS usually FATAL. Rabies cycle : Wolves, foxes, skunks , raccoons and bats serve as the natural reservoir for the virus . Dogs and cats serve as the most important sources of human infection , because of their close CNTACT with humans. TRANSMITION Animal Natural Reservoir all over the world are non-domesticated mammals ( wild animals) as(FOX,WOLVS,BAT) which accidentally bite domestic animals usually dogs ,cats The virus rarely affects rodents, rabbits or horses Humans usually become infected through DOG bites Human to human transmission is extremely rare. Natural reservoir for rabies (WILD ANIMALS). Natural reservoir for the virus . Source of human infection(DOMESTIC ANIMALS) INCUPATION PEROID The incubation period varies depending on the nature ,severity of the bite(virus concentration in the saliva of the animal) and on location of the bite (head ,neck ,trunk, extremities,foot,leg) Bites involving the head or neck has short The IP can be from 10 days (2 WEEKS 2 MONTHS) IP. ONE YEAR Pathogenesis of Rabies Structure and classification . Family : Rhabdoviridae . genus : lyssa virus The virus is bullet shaped, with helical nucleocapsid. The viral genome is ss –RNA. Virion contain the enzyme transcriptase. One major antigenic type exist in nature . Rabies virus . Pathogenesis . After entry the virus replicate s in muscles and connective tissue at the site of the bite . The virus then travels through peripheral nerves to the spinal cord and the brain . After replication in the CNS, the virus spreads through the peripheral nerves to the salivary sheds in saliva . glands and Clinical features The incubation period in human depends on the site of the bite( usually between 1 – 4 months ). A bite on the face or neck tend to produce a shorter incubation period than a similar wound on the foot or leg . Clinical features 1--The prodromal phase: malaise, headache, nausea , vomiting and fever . Usually there is discomfort or paresthesia at the site of the bite . 2--The excitement phase: anxiety, agitation, increased nervousness, hyper reactivity, pupillary dilation, increased salivation. painful laryngeal and pharyngeal spasms triggered by swallowing saliva ( hydrophobia ). Clinical features 3-- Paralytic phase : soon a wide variety of the CNS signs appear including hallucination, lack of coordination, mental confusion and paralysis . 4-- finally coma develop and death . Recovery from rabies is extremely rare. Only six documented cases of human survival from clinical rabies have been reported. . Laboratory diagnosis of rabies . In human or animals by detection of rabies virus –RNA in saliva, spinal cord ,brain tissue,cornea or skin by immuno flourecent or PCR . Detection of rabies antigen in skin biopsy from the neck, using direct immuno flourescent technique ( IF ). Laboratory diagnosis of rabies in animals . In dead animals: by detection of rabies antigens in brain tissue, using direct immuno-flourescent technique (IF ) . Or demonstration of Negri bodies , intra- cytoplasmic inclusion bodies, in the brain tissue. Negri bodies . Negri bodies Negri bodies Pre-exposure immunization Generally confined to those occupationally at risk such as veterinarians, animal holders, and long term visitors to endemic areas. 3-doses of the human diploid vaccine one month apart, with a booster They should be given dose two years later .Tow booster doses should be given if they are exposed to infection. Post –exposure or suspicion of exposure Active immunization : The main vaccine is the human diploid cell vaccine. Contains: inactivated virus disrupted into subunits. Prepared : in human embryo lung cell. Administered : intramuscularly in 5-doses spaced at 0, 3, 7, 14 & 30 days . Post- exposure, or suspicion of exposure The wound should washed thoroughly with soap and water and alcohol or iodine solution. Patients should be given combined passive and active immunization. Passive immunization: by injecting of human anti-rabies immunoglobulin( RIG ). The dose is 20 Iu/kg, half given around the bite wound and the other half intramuscularly Prevention Stray animals should be destroyed. Vaccination of pet dogs and animals should be mandatory A live attenuates vaccine is available for immunizing dogs and cats . Marburg and Ebola virus infection Marburg virus was discovered in 1967, when outbreaks of hemorrhagic fever occurred simultaneously in Marburg in Germany . Ebola virus was discovered in 1976 and named as a river in Congo (ZAIRE). They have been classified in the family filoviridae. They have ss-RNA genomes with negative polarity . Marburg and Ebola virus infections They cause hemorrhagic fever with multiple organs dysfunction and high mortality rate . They infect human and non-human primates (chimpanzees, gorillas and monkeys). ANIMAL to HUMAN transmission occur by a direct contact with blood , tissues & secretion from infected chimpanzees, gorillas and monkeys . HUMAN TO HUMAN transmission seems to be important in human outbreak via direct contact with infected blood, organs or secretion of infected person. Ebola virus . Pathogenesis Ebola & Marburg viruses micro- vascular cause damage to the resulting in increased vascular permeability, which may contributing to the hemorrhagic manifestation . Clinical features OF HAEMORRAGIC FEVER due to Marburg and Ebola virus. Short incubation period ranging from 5 – 10 days . The two viruses cause diseases with similar clinical picture . The disease is started with fever, myalgia, and headache Mucosal bleeding from nose, gum, conjunctiva, GIT and vagina Nausea, vomiting and diarrhea. CNS symptom: mental confusion and coma. Prognosis of HAEMORRAGIC FEVER due to Marburg and Ebola virus. . During the second week of infection the patient either begin recovery or develop fatal multiple organ failure. Mortality rate ranges from 25 Ebola. – 90 % , higher with Laboratory diagnosis Hemorrhagic fever viruses are classified as SAFTY level class BIO- 4 pathogens ( BSL-4 ) . Lab diagnosis must be accomplished under maximum biological containment conditions . The two most commonly used lab methods are, detection of the viral genome in the patient blood using PCR or isolation of the virus in tissue culture followed by identification of the virus by PCR . Treatment and prevention Treatment is supportive and some trial using INTERFERON and RIBAVIRIN seems promissing . There is no vaccine available yet for Ebola and Marburg infections. Rodent- transmitted viruses . 1– Lassa virus ( family: Arenaviridae). 2– Hantavirus ( family : Bunyaviridae ). Lassa fever Caused by Lassa virus was discovered in 1969, in Nigeria . ss-. RNA with negative polarity . Infected rodents shed the virus in their excreta (urine, saliva and feces ). Humans are infected by direct contact with rodents excreta, or eating foods contaminated with these excreta OR through inhalation of tiny particles contaminated with rodents excreta. Person to person transmission occurs by direct contact with infected blood and body fluids OR contaminated medical instruments . Clinical features of hemorrhagic fever (lassa virus) Incubation period: 6 – 21 days. Lassa fever is an acute viral hemorrhagic fever with multi-organ dysfunction (see before). About 80% of infected individuals show no symptoms or had mild illness . The remaining 20% develop severe multi-systemic disease and hemorrhage with high mortality rate. Lab diagnosis Must be accomplished under maximum biological containment conditions. Isolation of the virus in tissue culture, followed by identification of the isolated virus. Detection of the viral PCR . RNA in the patient blood using Treatment & prevention There is no specific anti-viral drug therapy. Treatment is supportive. Prevention: By controlling rodents. There is no vaccine available yet . Hantavirus The virus was named Hanta as the river Hantaan in Korea . Enveloped ,ss-RNA virus with negative polarity . The virus is excreted in the urine, saliva and feces of infected mice Humans are infected when they come in direct contact with rodents excreta . No Human to Human transmission has been recorded Hanta virus . Deer mouse Transmission of hanta virus . Types of diseases. Hantavirus infection ranges from mild to severe . Severe cases may take the form of: 1- Renal hemorrhagic syndrome ( RHS ). 2- Hanta pulmonary syndrome ( HPS ) . The initial symptoms of both syndromes are similar: Fever, headache, myalgia, nausea, vomiting, and abdominal pain . 1- Renal hemorrhagic syndrome ( RHS ) . Incubation period : 1-5 weeks. The disease ranges from mild cases to severe . The virus infects the endothelial cells of the blood vessels in the kidneys, leading to increase vascular permeability, decreased blood pressure and hemorrhage . In severe cases, there will be hemorrhage, renal dysfunction. Proteinuria and oliguria . 2- Hanta pulmonary syndrome ( HPS ). The disease ranges from mild cases to severe . The virus affects endothelial cells of the small blood vessels in the lungs, leading to increase vascular permeability, decreased blood pressure and hemorrhage . In severe cases, tachycardia, shortness of breath, dyspnea, pleural effusion and hemorrhage are developed . Mortality rate is about 50- 70 % . Lab diagnosis 1 -- By isolation of the virus in tissue culture, followed by identification of the isolated virus . 2– by detection of the PCR . viral – RNA , using Treatment & prevention. There is no specific anti-viral drug therapy. Treatment is supportive . There is no vaccine available yet .