* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Pediatric Cardiothoracic Surgery
Remote ischemic conditioning wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Electrocardiography wikipedia , lookup
Antihypertensive drug wikipedia , lookup
Heart failure wikipedia , lookup
Coronary artery disease wikipedia , lookup
Aortic stenosis wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Myocardial infarction wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Cardiothoracic surgery wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Congenital heart defect wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Atrial septal defect wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
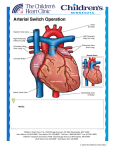
Pediatric Cardiothoracic Surgery Bjarni Torfason, dósent Yfirlæknir hjarta- og lungnaskurðdeildar Landspítalans Háskóli Íslands Læknadeild Cardiothoracic Surgery Undirsérgreinar: • • • • Adult Cardiac Surgery General Thoracic Surgery Pediatric Cardiothoracic Surgery Transplantation Pediatric Cardiothoracic Surgery • Thorax – – – – Veggur Pleura Lungu Mediastinum • Hjarta og æðar í thorax – Meðfæddir galla – Áunnir gallar/ sjúkdómar Pediatric Cardiothoracic Surgery • Thorax – – – – Veggur Pleura Lungu Mediastinum • Hjarta og æðar í thorax – Meðfæddir gallar – Áunnir gallar/ sjúkdómar Pectus carinatum Pectus excavatum Pectus excavatum - Nuss Pectus excavatum - Nuss • Viðmið: a/b > 3,2 Video 10:46 Spangartaka í ágúst 2007 3 árum frá Nuss reconstruction Pediatric Cardiothoracic Surgery • Thorax – – – – Veggur Pleura Lungu Mediastinum • Hjarta og æðar í thorax – Meðfæddir gallar – Áunnir gallar/ sjúkdómar Thorax Feb 2005 Vol 60 Suppl 1 www.brit-thoracic.org.uk BTS guidelines for the Management of Pleural Infection in Children Dr Ian Balfour-Lynn Royal Brompton Hospital 3 year old boy – 1w fever, malaise, cough, DIB IVABs no improvement so transferred Drain inserted, urokinase, IV cefuroxime B/C – Pneumococcus. Pleural fluid - sterile 4 days later – well but febrile, drain out & home next day Back to normal by 2 weeks 7 week follow up - Paediatric Pleural Diseases Subcommittee of the BTS Standards of Care Committee • • • • • • • • • Dr Ian Balfour-Lynn Paediatric Respiratory Medicine, Royal Brompton Hospital Dr Ed Abrahamson Paediatric A&E & General Paediatrics, Chelsea & Westminster Hospital Mr Gordon Cohen Pediatric Cardiothoracic Surgery, Seattle, USA Dr John Hartley Microbiologist, Great Ormond Street Hospital Dr Susan King Radiologist, Bristol Mr Dakshesh Parikh Paediatric Surgeon, Birmingham Dr David Spencer Paediatric Respiratory Medicine, Newcastle Dr Anne Thomson Paediatric Respiratory Medicine, Oxford Dr Donald Urquhart SpR North Thames Paediatric Respiratory Medicine Training Scheme New presentation Clinical suspicion parapneumonic effusion Pneumonia diagnosis Treatment failure at 48 hours Chest x-ray Pleural effusion? YES Confirm on chest ultrasound Refer to respiratory paediatrician Refer to respiratory paediatrician Suggestion of malignancy? Refer to respiratory paediatrician Suggestion of malignancy? Small volume diagnostic tap YES Refer to respiratory paediatrician Suggestion of malignancy? Small volume diagnostic tap YES NO Suggestion of infection? YES Intravenous antibiotics Refer to respiratory paediatrician Suggestion of malignancy? Small volume diagnostic tap YES NO Suggestion of infection? YES Intravenous antibiotics Medical option Early surgical option Medical option Insert chest drain Pleural fluid microbiology & cell diff. Echogenic or loculated on U/S? Thick fluid draining? YES Intrapleural fibrinolytics Medical option Insert chest drain Pleural fluid microbiology & cell diff. Echogenic or loculated on U/S? Thick fluid draining? YES Intrapleural fibrinolytics Early surgical option Consider chest CT scan VATS or Early mini-thoracotomy Medical option Early surgical option Insert chest drain Pleural fluid microbiology & cell diff. Echogenic or loculated on U/S? Thick fluid draining? Consider chest CT scan VATS or Early mini-thoracotomy YES Intrapleural fibrinolytics Is the patient better? (fluid drained and sepsis improved) Is the patient better? (fluid drained and sepsis improved) YES Remove tube Stop IV antibiotics Oral antibiotics 1-4 weeks Discharge & follow-up Is the patient better? (fluid drained and sepsis improved) NO Consult with paediatric thoracic surgeon re. late surgery Consider chest CT scan YES Remove tube Stop IV antibiotics Oral antibiotics 1-4 weeks Discharge & follow-up SIGN levels of evidence • I – meta-analyses, RCTs (incl. systematic reviews) I++, I+, I• II – case-control or cohort studies (incl. systematic reviews) II++, II+, II• III – case reports, case studies • IV – expert opinion SIGN grades of recommendations • A – evidence from meta-analysis, systematic review, RCT (I++ or applicable I+) • B – evidence from applicable II++ or extrapolated I++, I+ • C – evidence from applicable II+ or extrapolated II++ • D – evidence from III or IV SIGN ratings 107 46 120 50 100 40 80 30 60 40 13 22 23 I II III IV 7 4 10 20 0 20 0 0 A B C D Levels of evidence Grades of recommendations n=165 n=57 Clinical picture • All children with parapneumonic effusion or empyema should be admitted to hospital. [D] • If a child remains pyrexial or unwell 48 hours after admission for pneumonia, parapneumonic effusion / empyema must be excluded. [D] Diagnostic imaging • Postero-anterior or anteroposterior radiographs should be taken, there is no role for a routine lateral radiograph. [D] • Ultrasound must be used to confirm the presence of a pleural fluid collection. [D] • Chest CT scan should not be performed routinely. [D] Diagnostic analysis of pleural fluid • Aspirated pleural fluid should be sent for differential cell count. [D] • Tuberculosis and malignancy must be excluded in the presence of pleural lymphocytosis. [C] • Biochemical analysis of pleural fluid is unnecessary… [D] Referral to tertiary centre • A respiratory paediatrician should be involved early in the care of all patients requiring chest tube drainage for a pleural infection. [D] GOSH • Patients with chest drains should be managed on specialist wards by staff trained in chest drain management. [D] Conservative management (antibiotics ± simple drainage) • Effusions which are enlarging and / or compromising respiratory function should not be managed by antibiotics alone. [D] Repeated thoracocentesis • If a child has significant pleural infection then a drain should be inserted at the outset, and repeated taps are not recommended. [D] Antibiotics 1 • All cases should be treated with intravenous antibiotics and must include cover for S pneumoniae. [D] • Broader spectrum cover is required for hospital-acquired infections, as well as those secondary to surgery, trauma and aspiration. [D] Antibiotics 2 • • • • • • Cefuroxime Co-amoxiclav Penicillin and flucloxacillin Amoxicillin and flucloxacillin Clindamycin Discharge: oral co-amoxiclav 1- 4 wks Chest drains 1 • If GA is not being used, IV sedation should only be given by those trained in the use of conscious sedation, airway management & resuscitation of children, using full monitoring equipment. [D] • Ultrasound should be used to guide thoracocentesis or drain placement. [C] Chest drains 2 • Since there is no evidence that large bore chest drains confer any advantage, small drains (including pigtail catheters) should be used whenever possible to minimise patient discomfort. [C] • The drain should be clamped for 1 hour once 10 mls/kg are initially removed. [D] B Intrapleural fibrinolytics • Intrapleural fibrinolytics shorten hospital stay and are recommended for any complicated parapneumonic effusion (thick fluid with loculations) or empyema (overt pus). [B] • Urokinase should be given twice daily for 3 days (6 doses in total) using 40,000 units in 40 mls 0.9% saline for children aged 1 year or above, and 10,000 units in 10 mls 0.9% saline for children aged under 1 year. [B] Thomson et al Thorax 2002;57 343-7 Surgery • Failure of chest tube drainage, antibiotics and fibrinolytics should prompt early discussion with a thoracic surgeon. [D] • Patients should be considered for surgical treatment if they have persisting sepsis in association with a persistent pleural collection, despite chest tube drainage and antibiotics. [D] • Organised empyema in a symptomatic child requires formal thoracotomy and decortication. [D] Other management • Chest physiotherapy is not beneficial and should not be performed in children with empyema. [D] • Secondary thrombocytosis (platelet count >500 x109/L) is common but benign; antiplatelet therapy is not necessary. [D] Follow-up • Children should be followed up after discharge until they have recovered completely and their chest radiograph has returned to near normal. [D] • Underlying diagnoses – for example, immunodeficiency, cystic fibrosis – may need to be considered. [D] The messages • The evidence on which to base recommendations is poor / absent • Adult data are not transferable • This is a tertiary condition • Children with empyema almost always have an excellent outcome – whatever the management • Trials are needed… Pediatric Cardiothoracic Surgery • Thorax – – – – Veggur Pleura Lungu Mediastinum • Hjarta og æðar í thorax – Meðfæddir gallar – Áunnir gallar/ sjúkdómar Andnauð við fæðingu What is your differential diagnosis? • Congenital diaphragmatic hernia • Pneumothorax • Congenital cystic adenomatoid malformation (CCAM) • Pulmonary sequestration – Intrapulmonary – Extrapulmonary • Congenital Lobar Emphysema • Bronchogenic Cyst CDH NGT Treatment: CDH • Delayed surgical approach; not a surgical emergency • Conventional vent, Oscillator, “gentle ventilation”, possible ECMO (10-15%) – Goal is prevention of barotrauma • Primary repair; patch sometimes needed • Overall survival 50-80 % CDH • • • • Bochdalek: posterolateral defect; usually on left Morgagni: retrosternal (anterior); presents late Lung hypoplasia affects both sides. Pulmonary hypertension / persistent fetal circulation are the greatest challenges. • Most repairs do not necessitate a postoperative chest tube. Differential Diagnosis Congenital Lobar Emphysema: - isolated idiopathic hyperinflation of one lobe; respiratory difficulties often at birth or in infancy; worsens with time by air trapping Differential Diagnosis Pulmonary Sequestration: - a segment of lung without anatomic - bronchial communication, systemic arterial supply from thoracic or abdominal aorta “Extralobar”: often incidental “Intralobar”: found within normal lung parenchyma (lower lobes); prone to infection Differential Diagnosis Congenital Cystic Adenomatoid Malformation (CCAM) - solid/cystic lung malformation - Can present at prenatal U/S or resp distress at birth or with infection in first few years of life. If large, can cause fetal hydrops. Differential Diagnosis Bronchogenic Cyst - Cyst found in hilum, mediastinum, or within lung parenchyma. - Can compress airway and cause atelectasis, pneumonia, air trapping. Pediatric Cardiothoracic Surgery • Thorax – – – – Trauma Veggur Pleura Lungu Mediastinum • Hjarta og æðar í thorax – Meðfæddir gallar – Áunnir gallar/ sjúkdómar Pediatric Thorax Trauma + see last years lectures Tx Trauma Mechanism of Injury and Associated Mortality AGE 0-5 6-10 >10 Proportion 36% 27% 37% Mechanism MV=70.3% Peds=42.9% MV=61.9% Peds =16.6% MV=41.4% GSW=27.6% Struck=9.9% Bike=10.5 Bike=8.2% Stab=2.3% Sport=3.7% Fall=1.5% Fall=1.5% Fall=0.9% Sport=0.8% Organ Weight During Violent Impact at Various Velocities Weight (kg) 40 km/h 72 km/h 105 km/h HEART (0.35) BRAIN (1.5) 3.5 kg 14 kg 31.5 kg 15 kg 60 kg 135 kg Whole Body (70) 700 kg 2800 kg 6300 kg Mechanism of Injury by Age 7,000 6,000 5,000 4,000 MVC Violence Fall 3,000 2,000 1,000 0 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 36 38 40 42 44 46 48 50 52 54 56 58 60 62 64 66 68 70 72 74 76 78 80 82 84 86 88 1 3 5 7 9 11 13 15 17 19 21 23 25 27 29 31 33 35 37 39 41 43 45 47 49 51 53 55 57 59 61 63 65 67 69 71 73 75 77 79 81 83 85 87 89 Age (years) Figure 12A Number of patients injured by the most common mechanism of injury categories at each age from 0 to 89. Violence was defined for the following mechanisms of injury: GSW(gunshot wounds), stab wounds and assault/fight. Total N = 453,806. Mechanism of injury definitions in Appendix B. Prevalence of Pediatric Trauma • Trauma is the leading cause of death in infants and children • Trauma is the cause of 50% of deaths in people between 5 and 34 years of age • Motor vehicle related accidents account for 50% of pediatric trauma cases • $16 billion is spent annually caring for injuries to children less than 16 years of age Anatomic Characteristics of the Pediatric Patient and Significance to Trauma Care Variable Significance •Large volume of blood in head •Cerebral edema develops rapidly •Poor muscular support in neck •Flexion/extension injuries occur •Decreased alveolar surface area •Increased metabolic rate •Injury leads to rapid compromise •Decreased airway caliber •Increased airway resistance •Heart higher in chest, •Small pericardial sack •Prone to injury and cardiac tamponade •Thin walled, small abdomen •Organs not well protected •Bones soft and pliable •Fractures less common •Renal function not well developed •Prone to develop acute renal failure •Large body surface area •Prone to hypothermia Anatomic and Physiologic Differences Child/Adult • Pliable chest wall – Transmission of force within the thoracic skeleton to the pulmonary parenchyma • Mobility of the mediastinal structures – Vulnerability to tension pneumothorax and vascular disruptions Chest Trauma • 5 - 10 % of all injuries involve the chest • 2/3 chest injured children have injury in another organ system • thoracic injuries account for ¼ of pediatric trauma related deaths • Rib fractures are a marker of significant force and occur with < 50 % of chest trauma Chest Trauma Cont’d • PATTERN OF INJURY • Pulmonary contusion is common +/- direct intrapulmonary hemorrhage or pneumothorax, usually without rib fractures • RARE : diaphragmatic rupture, aortic transection, major tracheobronchial tears, flail chest, sternal fractures and cardiac contusion Chest Trauma Cont’d • 2nd leading cause pediatric trauma death • compliant chest wall ∴ rib fract uncommon – significant injuries w/o external signs – if rib fracture present, expect severe injury • treat conservatively: – 15% require more than chest tube • pulmonary contusion most common, aortic injury rare Chest Trauma Cont’d • Traumatic asphyxia – Sudden compression elastic chest wall against closed glottis→↑ intrathoracic pressure→obstruction of SVC/IVC→ capillary extravasation: petechiae face, neck ,chest, periorbital edema, retinal hemorrhages, resp distress, hemoptysis, pulmonary/cardiac contusions, liver injuries, pneumothorax • Treat: chest tube prn, ventilate, PEEP, elevate head Pediatric Cardiothoracic Surgery • Thorax – – – – Veggur Pleura Lungu Mediastinum • Hjarta og æðar í thorax – Meðfæddir gallar – Áunnir gallar/ sjúkdómar Cardiovascular Surgery Knee-chest Position Frumkvöðlar • 1952 Robert Edward Gross – Atrial trekt “Open heart surgery” ASD 0/3 • 1952 Floyd John Lewis – Hypothermia “Open heart surgery” ASD 9/11 • 1953 Dr. John Gibbon – CPB support ASD 1/5 • 1954 Dr. Walt Lillehei – Cross circulation VSD – Tetralogy of Fallot • 1952 Robert Edward Gross – Atrial well “Open heart surgery” ASD 0/3 • 1952 Floyd John Lewis – Hypothermia “Open heart surgery” ASD 9/11 • 1953 Dr. John Gibbon – CPB support ASD 1/5 • 1954 Dr. Walt Lillehei – Cross circulation VSD – Tetralogy of Fallot Hiti og kuldi • Hiti er merki um líf og bruna • Metabolisk vörn í náttúrunni: – Hibernation: dýr liggja í dvala yfir veturinn. – Animals seek cool environment when ischemic • ” kæling matvæla fyrirbyggir skemmdir. • Reynslan hefur kennt okkur að: – ”Ice on injuries reduces swelling and tissue damage” • Physiological and biochemical rationale – Tissue metabolic rates decrease as body temperature decreases • 1952 Robert Edward Gross – Atrial well “Open heart surgery” ASD 0/3 • 1952 Floyd John Lewis – Hypothermia “Open heart surgery” ASD 9/11 • 1953 Dr. John Gibbon – CPB support ASD 1/5 • 1954 Dr. Walt Lillehei Surface Cooling Limitations – Cross circulation VSD Surface cooling used in COOL AID feasibility trial – Tetralogy of Fallot demonstrated limitations of * surface cooling Target temperature of 32°C Actual temperatures reached as low as 28°C Wide range in actual temperatures and slow cooling times Intensive cooling regime including blankets, alcohol wipes with paralyzed and intubated patients * Stroke 2001; 32: 1847-54 Target Temperature Frumkvöðlar • 1952 Robert Edward Gross – Atrial well “Open heart surgery” ASD 0/3 • 1952 Floyd John Lewis – Hypothermia “Open heart surgery” ASD 9/11 • 1953 Dr. John Gibbon – CPB support ASD 1/5 • 1954 Dr. Walt Lillehei – Cross circulation VSD – Tetralogy of Fallot Frumkvöðlar • 1952 Robert Edward Gross – Atrial well “Open heart surgery” ASD 0/3 • 1952 Floyd John Lewis – Hypothermia “Open heart surgery” ASD 9/11 • 1953 Dr. John Gibbon – CPB support ASD 1/5 • 1954 Dr. Walt Lillehei – Cross circulation VSD – Cross circulation Tetralogy of Fallot • CPB – – – – – – – – – – svæfingalækningar Blóðflokkar EKG 1903 Positive pressure ventilation Rtg.1895 Contrast 1923 Hjartaþræðing Heparin 1915 Protamin Oral anticoagulants 1940 Roller pump • 1955 John W. Kirklin – teamwork • “Cardiac team” – – – – Cardiac Surgery Anesthesiology assoc. 1972 Perfusion technology Cardiac intensive care medicin • 1955 Dr. DeWall Bubble oxygenator • Nszih Zuhdi Hemodilution techniques • 1960 combined pump and hypothermia – Ca. 1.000.000 / Y / US • • • • • CABG,VALVES, CONG. NEUROSURG.. HEART/LUNG TRANSPL., THORAC.AORTA ECMO pH og CO2 Alpha stat – sbr,. Dvali dýra • Basiskt pH stat cerebral flæði súrt – Peter C. Laussen Paediatric Anaesthesia March 2002 1883 Theodore Bilroth ' The Surgeon who should attempt to suture a wound of the heart would lose the respect of his colleagues. Cardiac Surgery Reykjavik 2002 Cardiac Surgical output total: 243 cases Coronary Surgery 182 Valve 38 Congenit 16 Other:ECMO3,MAZE1,Aneurysms7, AICD12 etc.: 26 TMR CABG+Valve OPCAB CABG Fjöldi aðgerða 2005 2006 2007 CABG 120 108 133 OpCAB 45 22 28 Lokuaðg. 66 54 64 Hjartaviðg. 9 3 7 Ósæðaaðg. 12 12 6 ECMO 0 3 8 Gervilunga (iLA) 2 1 0 Hjálparhjarta 0 1 4 Aðrar HLV 7 1 8 22 27 39 259 200 246 IABP Fjöldi milli ára 140 120 133 120 108 Fjöldi 100 80 66 64 54 60 45 40 39 22 28 22 20 9 3 7 12 12 6 0 3 8 2 1 0 0 1 4 Gervilunga (iLA) Hjálparhjarta 7 1 27 8 0 CABG OpCAB Lokuaðg. Hjartaviðg. Ósæðaaðg. ECMO Meðferð 2005 2006 2007 Aðrar HLV IABP Neonatal Surgery Reykjavík Boston Risk Categories 1= ASD, PDA (>30 d) , coarct (>30 d) 2=ASD/VSD, TOF, Glenn, sub AS 3=AVR, Ross procedure, MVR 4=arterial switch, Truncus arteriosus 5=truncus and interrupted arch 6= Norwood, Damus-Kaye-Stansel Congenital Disease • • • • • Patent Ductus Arteriosus Aortic Coarctation Atrial Septal Defect Ventricular Septal Defect Valvular Heart Disease – Ross Procedure Open Heart Surgery • • • • • Management of chest /thoracotomy tubes Risk for Infection Risk for Hypo/Hyperthermia Risk for Fluid Volume Overload Complications – – – – – – – – Hemmorhage Shock Heart Block CHF Post Cardiac Surgery Syndrome Post Perfusion Syndrome Infection Atelectasis ECC : Venous (IVC and SVC) Cannulae Aortic Cannula Oxygenator Pump Heater/Cooler CardioPulmonary Bypass Hjartastarfsemin Samhæfing PM Indian Pacing Electrophysiol. J. 2003;3(1):23 Video: Pacemaker 1:20 Video Pacemaker • Usually placed sub-xyphoid or mid to lower abd • Teach parents how to take child’s pulse daily • Batteries can last up to15 years Nýgengi hjartagalla • 1 in every 120 births has CHD • Mild – resolve by themselves • Moderate –Non life threatening – but require treatment • Severe – multiple operations , lifetime medication Pediatric Cardiac Disease • • • • Congenital Cyanotic: 22% Acyanotic: 68% Acquired: 10% – – – – – • Algengustu Congenital Kawasaki disease Rheumatic Tubercular Collagen Endocarditis – – – – – – VSD ASD PDA TOF PS AS 25% 6% 6% 5% 5% 5% Ceylon Med J 2001 Sep; 46 (3): 96-8; Indian J Pediatr. 2001 Aug;68 (8):757-7 Nelson’s Textbook of pediatrics; 17 ed. Common acyanotic lesions • • • • • • • • • • Ventricular septal defects Atrial septal defects Atrio-ventricular septal defects Patent ductus arteriosus Truncus arteriosus Pulmonary stenosis Aortic stenosis Mitral stenosis/incompetence Coarctation of aorta Tricuspid regurgitation Common Cyanotic Lesions Decreased flow 1. Tetralogy of Fallot 2. Tricuspid Atresia 3. Severe Pulmonic Stenosis 4. Ebstein’s anamoly Increased Flow 5. Transposition of the great vessles 6. VSD with pulmonary atresia Common Lesions “producing” cyanosis 7. Truncus Arteriosus 8. Hypoplastic left heart 9. Single ventricle 10.TAPVR with infradiaphragmatic obstruction Heart Transplant • Pre and Post-Op same as any heart surgery • Long-term Consequences – Atherosclerosis – Lifetime use of immunosuppressant drugs – Number one complication is Rejection • Hyperacute • Acute • Chronic Classification of Congenital Heart Disease • Eldri – Acyanotic – Cyanotic • Nýrri – Increased Pulmonary Blood Flow – Obstructed Blood Flow – Mixed Blood Flow – Decreased Pulmonary Blood Flow Timing and Type of Surgery • Cardiac catheterization procedures – Balloon atrial septostomy – Balloon valvuloplasty – Balloon angioplasty • Open versus Closed • Palliative versus Corrective – Trend towards early, corrective surgery, even in preterm or low birth weight infants Types of Closed Heart Surgery • Patent Ductus Arteriosus • Coarctation of the Aorta • Blalock-Taussig Shunt Types of Open Heart Surgery Septal Defects: • Atrial Spetal Defect • Ventricular Septal Defect • Atrioventricular Septal Defect Defects Causing An Obstruction to Blood Flow: • Pulmonary Stenosis • Aortic Stenois • Tetralogy of Fallot Defects Involving The Great Arteries • Transpostion of the Great Arteries (Arterial Switch) Others: • Hypoplastic Left Heart Syndrome(Norwood operations) • Aortic Valve Replacement (Ross Procedure) Cardiac disease with normal/decreased vasculature • • • • • • • • Viral myocarditis Tetralogy of Fallot Pulmonary atresia Tricuspid atresia Endocardial fibroelastosis Aberrant left coronary artery Cystic medial necrosis Diabetic mother (http://www.emedicine.com/emerg/topic28.htm) Pediatric Cardiothoracic Surgery • Thorax – – – – Veggur Pleura Lungu Mediastinum • Hjarta og æðar í thorax – Meðfæddir gallar – Áunnir gallar/ sjúkdómar Congenital Disease • • • • • Patent Ductus Arteriosus Aortic Coarctation Atrial Septal Defect Ventricular Septal Defect Valvular Heart Disease – Ross Procedure Patent Ductus Arteriosus • Small defect no symptoms. • Large defect: – – – – – Wide pulse pressure Enlarged heart Thrill in L second IS Continuous murmur X-ray: prominent pulmonary artery with increased vascular markings. Patent Ductus Arteriosus Acyanotic Heart Defect – PDA • • • • Incidence 5-10% CHD Hemodynamic Diagnosis Management – Med – Surgical Mort <1% Video: Ductus 2:43 Aortic Coarctation Acyanotic Heart Defect Adult Týpa Aorta Ductus Aorta Neonatal Týpa Coarctation of Aorta • COA • 7 % of defects • Congenital narrowing of the descending aorta • 80% have aortic-valve anomalies (Bicusp) • Difference in BP in arms and legs (severe obstruction) Diagnosis and Treatment • In 50% the narrowing is not severe enough to cause symptoms in the first days of life. • When the PDA closes a higher resistance develops and heart failure can develop. • Pulses in the groin and leg will be diminished • Echocardiogram will show the defect in the aorta Treatment • Prostaglandin may be given to keep the PDA open to reduce the pressure changes • The most common repair is resection of the narrowed area with re-anastomosis of the two ends • Surgical complications – kidney damage due to clamping off of blood flow during surgery • High blood pressure post surgery – may need to be on antihypertensives • Antibiotic prophylactic need due to possible aortic valve abnormalities. Coarctation of the Aorta Acyanotic Heart Defect • • • • Incidence 8% CHD Hemodynamic Diagnosis Management – Med – Surgical Mort <5% » Ath mun á nýbura- og fullorðinspresentation Extended end to end eða subclavian flap reconstruction Aortic Coarctation Video 2 4:51 Atrial Septal Defects Acyanotic Heart Defects • Types of ASD: Atrial Septal Defects: secundum • Most common form of ASD (fossa ovalis) • In large defects, a considerable shunt of oxygenated blood flows from the left to the right atrium. • Mostly asymptomatic • The 2nd heart sound is characteristically widely split and fixed. Secundum Atrial Septal Defects:primum • Situated in the lower portion of the atrial septum and overlies the mitral and tricuspid valves. In most instances, a cleft in the anterior leaflet of the mitral valve is also noted. • Combination of a left-to-right shunt across the atrial defect and mitral insufficiency • C/F similar to that of an ostium secundum ASD Atrial Septal Defect • • • • • ASD 6-10% of defects Blood in left atrium flows into right atrium Pulmonary hypertension Reduced blood volume in systemic circulation • If left untreated may lead to pulmonary hypertension, congestive heart failure or stroke as an adult. Diagnosis and Treatment • Diagnosis: heart murmur may be heard in the pulmonary valve area because the heart is forcing an unusually large amount of blood through a normal sized valve. • Echocardiogram is the primary method used to diagnose the defect – it can show the hole and its size and any enlargement of the right atrium and ventricle in response to the extra work they are doing. Treatment • Surgical closure of the atrial septal defect • After closure in childhood the heart size will return to normal over a period of four to six months. • No restrictions to physical activity post closure Atrial Septal Defect • Enlargement of the right ventricle • Enlargement of atrium • Large pulmonary artery • increased pulmonary vascularity is. Atrial Septal Defects • Secundum ASDs are well tolerated during childhood. • Antibiotic prophylaxis for isolated secundum ASDs is not recommended. • Surgery closure is advised for all symptomatic patients and also for asymptomatic patients with a Qp:Qs ratio of at least 2:1. • Secundum defects are approached surgically but some secundum defects can be closed by transcatheter device • Ostium primum defects are approached surgically • Sinus venosum defects are approached surgically Atrial Septal Defects Acyanotic Heart Defects – ASD • • • • Incidence 5-10% CHD Hemodynamic Diagnosis Management – Med – Surgical Mort <1% Atrial Septal Defect ASDHjartagallar video\ASD.AVI Video: 6:44 Ventricular septal Defects • Small VSD – Asymptomatic – A loud, harsh, or blowing holosystolic murmur. • Large VSD – dyspnea, feeding difficulties, poor growth, profuse perspiration, recurrent pulmonary infections, and cardiac failure in early infancy. 80% Ventricular Septal Defect (VSD) Small VSDs, the chest radiograph is usually normal Large VSD: The presence of right ventricular hypertrophy, olegeimic lung fields (pulmonary hypertension or an associated pulmonic stenosis), gross cardiomegaly with prominence of both ventricles, the left atrium. Ventricular Septal defects • 30–50% of small defects close spontaneously, most frequently during the 1st 2 yr of life. • Small muscular VSDs are more likely to close (up to 80%) than membranous VSDs are (up to 35%). • infants with large defects have repeated episodes of respiratory infection and heart failure despite optimal medical management. • Surgical repair prior to development of an irreversible increase in pulmonary vasculalr resistance (usually prior to the patient's second birthday). Ventricular Septal Defect • • • • • • VSD 25-30% of defects Opening in the ventricular septum Left-to-right shunt Right ventricular hypertrophy Deficient systemic blood flow VSD • Small holes generally are asymptomatic • Medium to moderate holes will cause problems when the pressure in the right side of the heart decreases and blood will start to flow to the path of least resistance (from the left ventricle through the VSD to the right ventricle and into the lungs) • This will generally lead to CHF Diagnosis and Treatment • Diagnosis – heart murmur – clinical pearl a louder murmur may indicate a smaller hole due to the force that is needed for the blood to get through the hole. • Electrocardiogram – to see if there is a strain on the heart • Chest x-ray – size of heart • Echocardiogram – shows size of the hole and size of heart chambers Treatment VSD • CHF: diuretics of help get rid of extra fluid in the lungs • Digoxin if additional force needed to squeeze the heart • FTT or failure to grow may need higher calorie concentration • Will need prophylactic antibiotics before dental procedures if defect is not repaired Surgical Repair • Over a period of years the vessels in the lungs will develop thicker walls – the pressure in the lungs will increase and pulmonary vascular disease • If pressure in the lungs becomes too high the un-oxygenated blood with cross over to the left side of the heart and un-oxygenated blood with enter the circulatory system. • If the large VSD is repaired these changes will not occur. Acyanotic Heart Defects – VSD • • • • Incidence 20-25% CHD Hemodynamic Diagnosis Management – Med – Surgical Mort <5% Pulmonary artery banding Ventricular Septal Defect Video: VSD 2:34 Aortic Valve disease Acyanotic Heart Defects – AS • • • • Incidence 5% CHD Hemodynamic Diagnosis Management – Med – Surgical Mort 1-2%%, infant 15-20% http://www.hsforum.com/stories/storyReader$1469 • Ross Video: Ross 10:22 Tetralogy of Fallot (TOF) • 5-6% of defects • Most common cardiac malformation responsible for cyanosis in a child over 1 year TOF • Four Components – VSD – Pulmonary stenosis – narrowing of pulmonary valve – Overriding of the aorta – aortic valve is enlarged and appears to arise from both the left and right ventricles instead of the left ventricle – Hypertrophy of right ventricle – thickening of the muscular walls because of the right ventricle pumping at high pressure Clinical Manifestations • Depends on degree of right ventricular outflow tract obstruction. • Right-to-left shunt • Clubbing of digits • “tet” spells – flexing knees forward and upward • Irritability due to low oxygen levels Diagnosis • Cyanosis • Oxygen will have little effect on the cyanosis • Rtg • Loud heart murmur • Echocardiogram – demonstrates the four defects characteristic of tetralogy Treatment • If oxygen levels are extremely low prostaglandins may be administered IV to keep the PDA open • Complete repair is done when the infant is about 6 months of age • Palliation with BT shunt may be needed • Correction includes – Closure of the VSD with dacron patch – The narrowed pulmonary valve is enlarge – Hypertrophy of right heart should remodel within a few months when pressure in right side is reduced TOF – TOF • • • • Incidence 5-6% CHD Hemodynamic Diagnosis Management – Med – Surgical Mort varies + 5-10/2POY Palliative Procedures for TOF Current Indications Neonates with severe pulmonary artery atresia Aberrant course of the LAD from the RCA Procedures Performed •Blalock-Taussig •Modified Blacock Taussig •Central •Potts •Waterston Disadvantages of Palliation •Pulmonary artery distortion •Ventricular volume loading •Increased surgical risk from additional thoracotomy Most centers recommend primary surgical correction as the preferred procedure since mortality is very low. Recent series have reported down to 0% hospital mortality. www.ctsnet.org/doc/4954 BT, Modified BT Cyanotic Heart Defects – PA • • • • • Incidence <1% CHD Hemodynamic Manifestation Diagnosis Management FONTAN – Med – Surgical septostomy,Blalock-Taussig,valvotomy: » Mort: 10-25%, OutflTractReconstr 25% » Fontan <40% Cardiac disease with increased vasculature • • • • • • • Atrioventricular septal defects Congestive cardiac failure Transposition of great arteries with VSD Total anomalous pulmonary venous drainage Truncus arteriosus Single ventricle without pulmonary stenosis Hypoplastic left heart syndrome Cyanotic Heart Defects – TA • • • • • Incidence <1% CHD Hemodynamic Manifestation Diagnosis Management – Med – Surgical, » Mort 30%, » Rastelli´s Mort20-60% Cyanotic Heart Defects – TGA-Complete Transposition of the GA • • • • • Incidence 5% CHD Hemodynamic D-TGA Manifestation Diagnosis Management - Cardiac emergency – Med septostomycatlab/open Mort 10-25% 90%/1y » Surgical » Ventricular-level: Rastelli, or » Arterial-level Jatene´s, or » atrial Senning, Mustard´s TGA: Arterial-level Jatene´s Jatene operation for transposition of the great arteries • • • • Median sternotomy By-Pass is initiated The Heart is arrested Ligation of ductus arteriosus • Aorta is transected distal to the commissures • The pulmonary artery is transected • The coronary arteries are removed with a cuff of aortic wall (button). • Then the coronary arteries are sutured to the pulmonary stump creating a new (neo-) aortic stump • The aortic artery stump is then passed posterior to the pulmonary bifurcation (PA) and sutured to the reconstructed arterial stump of the left ventricle outflow tract (the LeCompte maneuver). Lastly, the pulmonary artery is joined to the new pulmonary artery stump creating normal circulatory anatomy and physiology TGA: Ventricular level Rastelli TGA: Atrial-level Senning/Mustard Cyanotic Heart Defects – Hypoplastic Left Heart Syndrome • • • • • Incidence 1-2% CHD Hemodynamic Manifestation Diagnosis Management – Med » Surgical NorwoodI:mixingII:FontanRA-PA MorIt 75% II50% » NWIII,IV » Transplantation?? Cyanotic Heart Defects – Total Anomal Pulm Venous Return • • • • • Incidence 1% CHD Hemodynamic PV-RA Manifestation Diagnosis Management – Med Balloon septostomy » Surgical Mort 10-25% Pediatric Cardiothoracic Surgery • Thorax – – – – Veggur Pleura Lungu Mediastinum • Hjarta og æðar í thorax – Meðfæddir gallar – Áunnir gallar/ sjúkdómar Pericardial Effusion • Presenting complaint – – – – – – – Precordial pain Cough Dyspnoea Abdominal pain Vomiting Fever Other organs involvement • Signs: – – – – – – – – Position: leaning forward. Puffy face Friction rub Absent apical impulse Muffled heart sounds Pulsus paradoxus Distended neck veins Low QRS complex, T inversion Pericardial Effusion • A relatively large pericardial effusion must be present to cause an enlarged cardiac shadow with the usual “water bottle” configuration on a chest roentgenogram Video: Chilothorax 8:56 Infective Endocarditis Presenting symptoms and clinical features include: • • • • • • • • • • • Fever Malaise Fatigue Anorexia Weight loss Splenomegaly Cardiac murmur Petechiae Roth spots Janeway lesions Osler nodes Some of the more diagnostic symptoms (the latter half of the above list) are occurring less frequently in patients with subacute IE, making diagnosis a greater challenge. Petechiae © 2007 About, Inc Roth Spots Janeway Lesion Osler Nodes © 2005 The Regents of the University of California The figure on the left is of a mitral valve vegetation shown by echocardiogram. The figure to the right shows one portion (called a leaflet) of the mitral valve. Cabell, C. H. et al. Circulation 2003;107:e185-e187 Copyright ©2003 American Heart Association Class I Indications for Surgery • Acute AR or MR with heart failure. • Acute AR with tachycardia and early closure of the MV. • Fungal endocarditis. • Annular or aortic abscess. • Sinus or aortic aneurysm. • Persistent bacteremia and valve dysfunction – After 7-10 days of appropriate antibiotics. Circulation. 98(18):1949-1984, 1998 Other Indications for Surgery • Class IIa • Class III – Recurrent emboli after appropriate abx. – Agent with known poor response to abx (GNR) with valve dysfunction. • Class IIb – Mobile vegetations >10 mm. Circulation. 98(18):1949-1984, 1998 – Early infections of MV that can likely be repaired. – Persistent pyrexia and leucocytosis with negative blood cultures. Congestive Cardiac Failure • Enlarged heart • Plethoric lung fields specially at bases Heart Failure • Biventricular pacing: Adults, but children? Heart Failure • ECMO and Counterpulsation IABP, Axial flow pump Impella, HMII Heart Failure ECMO 1:53 Video: ECMO Heart Failure • Bridge to improvement • Bridge to transplantation • Destination therapy Impella Animation Impella inn HMII nám Heart Failure • Heart transplantation Organ Transplantation in the United States 1 Jan 1988 – 31 Jan 2005 Organ Number Kidney Liver Pancreas Intestine Heart Lung 201,846 69,057 16,674 977 36,764 13,322 Total 338,640 www.unos.org Types of Lung Recipients • Pulmonary fibrosis • Emphysema/Alpha-1 antitrypsin deficiency • Pulmonary hypertension – Primary – Congenital heart disease • Cystic fibrosis ADULT LUNG TRANSPLANTATION Indications By Year (Number) 1250 Cystic 70 Fibrosis IPF Emphysema A1A Number of Transplants 60 1000 50 40 750 30 500 20 10 250 0 Myopathy 0 PPH 1982 1983 1984 1985 1986 1987 1988 1989 1990 1991 1992 1993 1990 1991 1992 1993 1994 1995 1996 1997 1998 1999 2000 2001 2002 2003 ISHLT Transplant Year Many variables account for the dramatic progress in outcomes of CHD • Better understanding of anatomy, embryology, genetics, pathophysiology, and natural history • Improved diagnosis • Support technology (e.g., cardiorespiratory support and monitoring technology in the OR and CICU, ECMO, mechanical assist devices) • Pharmacotherapy (e.g., pressors, ACE inhibitors, β-blockers, NO, Sildenofil, Bosentan) • Surgical techniques • Transcatheter therapy Tal Geva 2/04 • Organ transplants in Icelandic patients (1998 2007) 2008 Heart – 7 transplants in 6 patients – 5 are alive • Heart-lung – 4 transplants – 2 are alive • Lung – 6 transplants – 4 are alive • Kidney – 187 transplants in 167 patients – 110 transplants were from live donors and 77 from deceased donors – 111 are alive, 105 with a functioning transplant • Liver – 29 transplants in 26 patients – 5 children recieved a live donor transplant – 18 are alive Organ transplants from deceased donors in Icelandic patients, 1998-2007 1998 1999 2000 2001 2002 2003 2004 2005 2006 200 7 Heart Kidney Liver Lung *Same patient 2 1 1 2 3 2 1 1 1 1 2* 3 2 2 Total 2 3 6 2 21 2 3 2 15 1 1 4 Frekari lesning Common types of congenital heart defects Congenital heart defects are abnormalities that develop before birth. They can occur in the heart's chambers, valves or blood vessels. A baby may be born with only one defect or several that tend to occur in combination. Of the dozens of heart defects, some are mild and may need minimal or no medical treatment even through adulthood, while others are life-threatening, either immediately to the newborn or over time. Here's a look at some of the more common congenital heart defects. Compare them to the NORMAL HUMAN HEART, SHOWN HERE Congenital Heart Disease Type of Defect Mechanism Ventricular Septal Defect (VSD) There is a hole within the membranous or muscular portions of the intraventricular septum that produces a left-to-right shunt, more severe with larger defects Atrial Septal Defect (ASD) A hole from a septum secundum or septum primum defect in the interatrial septum produces a modest left-to-right shunt Patent Ductus Arteriosus (PDA) The ductus arteriosus, which normally closes soon after birth, remains open, and a left-to-right shunt develops Tetralogy of Fallot Pulmonic stenosis results in right ventricular hypertrophy and a right-to-left shunt across a VSD, which also has an overriding aorta Transposition of Great Vessels The aorta arises from the right ventricle and the pulmonic trunk from the left ventricle. A VSD, or ASD with PDA, is needed for extrauterine survival. There is right-to-left shunting. Truncus Arteriosus There is incomplete separation of the aortic and pulmonary outflows, along with VSD, which allows mixing of oxygenated and deoxygenated blood and right-to-left shunting Hypoplastic Left Heart Syndrome There are varying degrees of hypoplasia or atresia of the aortic and mitral valves, along with a small to absent left ventricular chamber Coarctation of Aorta Either just proximal (infantile form) or just distal (adult form) to the ductus is a narrowing of the aortic lumen, leading to outflow obstruction Total Anomalous Pulmonary Venous Return (TAPVR) The pulmonary veins do not directly connect to the left atrium, but drain into left innominate vein, coronary sinus, or some other site, leading to possible mixing of blood and right-sided overload Ventricular septal defect Sometimes called a hole in the heart, this defect — the most common congenital heart defect — occurs when the septum, the muscular wall separating the right and left ventricles, fails to fully form. The hole allows oxygen-rich blood to leak from the left ventricle into the right ventricle, instead of moving into the aorta and on to the body. Too much blood may flood the lungs. This defect can lead to heart failure, excessive blood pressure in the lungs (pulmonary hypertension), infections of the heart (endocarditis), irregular heart beats (arrhythmias) and delayed growth. Small holes may heal on their own or cause no symptoms. Larger holes may require surgical repair by stiching together or covering with a patch. Atrial septal defect Similar to a ventricular septal defect, this is a hole that occurs when the septum separating the right and left atria doesn't close properly. This allows blood from the left atrium to flow into the right atrium, instead of into the left ventricle and on to the aorta and the rest of the body. The defect can cause several complications, including arrhythmias, heart failure, stroke and, in rare cases, pulmonary hypertension. Minor cases may cause no symptoms and may not require treatment. Larger defects may require surgical closure or cardiac catheterization. Patent ductus arteriosus Before birth, a temporary blood vessel called the ductus arteriosus connects the pulmonary artery and the aorta. This allows blood to bypass the lungs because oxygen is delivered to the fetus through the placenta and umbilical cord. The temporary vessel normally closes within a few hours or days of birth since the lungs take over. If it remains open (patent), some blood that should circulate through the body is misdirected to the lungs. This defect can cause heart failure or endocarditisse heart failure or endocarditis. In infants, it can be closed with medications. In older children and adults, plugs, coils or surgery can be used to close the vessel. Pulmonary stenosis In this condition, the flow of blood from the right ventricle to the pulmonary artery is obstructed by narrowing at the pulmonary valve. When there's an obstruction (stenosis), the right ventricle must pump harder to get blood into the pulmonary artery. The defect may occur along with other defects, such as thickening of the muscle of the right ventricle immediately below the valve. In many cases, pulmonary stenosis is mild and doesn't require treatment. But because it can cause heart failure, arrhythmias or enlargement of the right heart chambers, surgery may be necessary to repair the stenosis or replace the valve. Special balloons to widen the valve (balloon valvuloplasty) may also be used. Tetralogy of Fallot This defect is a combination of four (tetralogy) congenital abnormalities. The four defects typically are ventricular septal defect (VSD), pulmonary stenosis, a misplaced aorta and a thickened right ventricular wall (right ventricular hypertrophy). They usually result in an insufficient amount of oxygenated blood reaching the body. Complications of tetralogy of Fallot (fuhLOE) include cyanosis — sometimes called "blue baby syndrome," since the lips, fingers and toes may have a bluish tinge from lack of oxygen — as well as poor eating, inability to tolerate exercise, arrhythmias, delayed growth and development, and stroke. Surgical repair of the defects is required early in life. Transposition of the great vessels (arteries) With this defect, the positions of the aorta and the pulmonary artery (the great arteries) are reversed (transposed). The aorta arises from the right ventricle instead of the left and the pulmonary artery arises from the left ventricle instead of the right. This creates a circulatory pattern that prevents nourishing oxygenated blood from reaching the body. This condition would quickly be fatal to a newborn except it's generally accompanied by another defect — commonly a septal defect or patent ductus arteriosus — that does allow oxygen-rich blood to get to the body. Surgical repair is usually necessary shortly after birth. Truncus arteriosus This is a defect in which the normally distinct pulmonary artery and aorta merge into one single great vessel (truncus) arising from the right and left ventricles. In addition, there's usually a large ventricular septal defect, essentially turning the right and left ventricles into a single chamber. This allows oxygenated and unoxygenated blood to mix. Too much blood may flow to the lungs, flooding them and making it difficult to breathe. It can also result in lifethreatening pulmonary hypertension. Surgery is needed to close the septal defect with a patch and to separate the pulmonary arteries from the trunk. A conduit is placed to connect the right ventricle to the pulmonary artery. Because the conduit doesn't grow with the child, repeat surgery may be necessary over time Hypoplastic left heart syndrome In this condition, the left side of the heart is underdeveloped (hypoplastic), including the aorta, aortic valve, left ventricle and mitral valve. As a result, the body doesn't receive enough oxygenated blood. In the first few days after a baby is born, the ductus arteriosus remains open (patent), allowing normal circulation, so the baby may seem fine initially. But when the ductus arteriosus naturally closes, signs and symptoms begin, including a bluish cast to the skin from lack of oxygen, difficulty breathing and poor feeding. This condition may be accompanied by an atrial septal defect. Treatment options for this life-threatening condition are a heart transplant or a multistage surgical procedure done during the first few years of life. Coarctation of the aorta This is a narrowing (coarctation), or constriction, in a portion of the aorta. Coarctation forces the heart to pump harder to get blood through the aorta and on to the rest of the body. This defect can cause several life-threatening complications, including severe hypertension, aortic aneurysm, dissection or rupture, endocarditis, brain hemorrhage, stroke, heart failure and premature coronary artery disease. Repair is typically recommended before age 10, either by surgically removing the affected portion or widening it through balloon angioplasty and placement of a stent. Aortic stenosis This is a defect that narrows or obstructs the aortic valve opening, making it difficult for the heart to pump blood into the aorta. Mild cases may not have symptoms initially, but they can worsen over time. The defect can cause heart enlargement, left-sided heart failure, arrhythmias, endocarditis and fainting. Treatment includes surgical repair or replacement of the valve or, in young children, widening through balloon valvuloplasty. If there's an extra conduction pathway, the electrical signal may arrive at the ventricles too soon. This condition is called Wolff-Parkinson-White syndrome (WPW). It's in a category of electrical abnormalities called "pre-excitation syndromes." Ebstein's anomaly This is a defect of the tricuspid valve, which controls blood flow between the heart's right atrium and right ventricle. The valve is positioned lower than normal into the ventricle instead of remaining between the atrium and the ventricle. Consequently, the ventricle is too small and the atrium too large, and neither functions properly. The valve is also malformed, often allowing blood to leak from the ventricle into the atrium. This defect often occurs along with other heart defects, including patent foramen ovale, atrial septal defect or WolffParkinson-White syndrome. Severe cases are life-threatening. Milder cases may have no signs or symptoms until adulthood. Treatment is with medications or with surgery to repair or replace the tricuspid valve, as well as treatment of associated conditions. Atrioventricular canal defect This is a combination of defects, including a large hole in the center of the heart and a single common valve instead of the separate tricuspid and mitral valves. Also called atrioventricular septal defect, this defect is classified by whether it's only partial, involving only the upper chambers of the heart, or complete, in which blood can travel freely among all four chambers of the heart. Both forms allow extra blood to circulate to the lungs, causing the heart to enlarge. The condition is often associated with Down syndrome. Infants may also have trouble breathing and not grow well. Surgery is often done in infancy to close the hole and reconstruct the valves. References • Text – Rogers: Textbook of Pediatric Intensive Care – Critical Cardiac Disease of Infants and Children • On-line – Picubook.net – Pedi-heart web-site – http://www.cincinnatichildrens.org/health/heart -encyclopedia/default.htm Complications ComplicationsofofParenteral ParenteralNutrition Nutrition --Technical Technical • • Placement complications – Pneumothorax – Arterial lacerations – Hemothorax – Mediastinal hematoma – Nerve injury Late complications – Erosion of catheter – Subclavian thrombosis – Septic thrombosis – – – – – Sympathetic effusion Thoracic duct injury Air embolism Hydrothorax Catheter embolism POTraC POTraC 2000 2000 Complications ComplicationsofofParenteral ParenteralNutrition Nutrition --Metabolic MetabolicComplications Complications • • • • Plasma electrolyte abnormalities Trace mineral deficiency – zinc, copper, chromium, selenium Essential fatty acid deficiency Disorders of glucose metabolism – Hypoglycemia – Hyperglycemia – Diabetic patient; hyperosmolar nonketotic coma – Liver function derangements POTraC POTraC 2000 2000 Complications ComplicationsofofParenteral ParenteralNutrition Nutrition ––Septic SepticComplications Complications • • Catheter Infection 1. Absence of proocol 2. Degree of colonization of the pericatheter skin; > 103 3. G(+) organism from remote site seeding the fibrin sleeve along catheter; vs G(-) organism 4. Candida from the gut Management of patient with suspected catheter sepsis POTraC POTraC 2000 2000 Prevention PreventionofofCatheter CatheterComplications Complications • Catheter Placement