* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Jon Magnuson, Glenn Fryxell, Linda Lasure, Doug Elliot (PNNL)
Survey
Document related concepts
G protein–coupled receptor wikipedia , lookup
Protein (nutrient) wikipedia , lookup
Biochemistry wikipedia , lookup
Protein adsorption wikipedia , lookup
Bottromycin wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Protein moonlighting wikipedia , lookup
Biosynthesis wikipedia , lookup
Western blot wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Metalloprotein wikipedia , lookup
Proteolysis wikipedia , lookup
Transcript
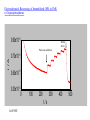
Immobilized Enzymes in Functionalized Nanoporous Materials Exhibit Enhanced Activity and Stability Eric J. Ackerman, Chenghong Lei,Yongsoon Shin (PNNL) Jon Magnuson, Glenn Fryxell, Linda Lasure, Doug Elliot (PNNL) Jun Liu (PNNL, now at Sandia) Hydrolysis of Organophosphorus by Immobilized OPH Stable enzymes entrapped in nanopores may one day be routinely used for chemical reactions. Enzymes in this environment are stable for extended periods of time. J. Am. Chem. Soc. 2002, 124, 11242−3 Potential Applications Enzymes are nano-machines of cells, catalyzing thousands of useful chemical reactions. Microscopic reversibility means that outside cells, reactions A --> B and B --> A are feasible. Unlike typical chemical catalysts, enzymatic reactions occur at ambient conditions; i.e. green technology. Enzyme fragility has been a primary limiting factor in applications. Our breakthrough is applicable at multiple scales: sensors to industrial reactions Focus areas: homeland security energy Why is this a breakthrough? Decades of work immobilizing enzymes has yielded small amounts of mostly inactive enzyme. Previous approaches generally destroyed the enzymes activity as a consequence of the immobilization procedure. This occurred either by killing the enzyme or burying it inside a material so that substrates and products could not enter and leave. Specific activity (enzyme activity per amount of enzyme) is the important parameter. We immobilize larger quantities of active enzyme per amount of material than other methods. Our immobilized enzyme exhibits enhanced stability and, for the first time, enhanced activity. Maintaining and Promoting Enzyme Activity Confinement can eliminate some expanded configurations of the unfolded chain, shifting the equilibrium from the unfolded state toward the native state. Denatured Enzyme, unfolded state in solution Renatured Enzyme, native state in a confined space Biochemistry 2001, 40: 11289-11293. Confined space: Mesoporous silica 60 nm 300 Å Mesoporous Silica Confined space for enzyme (protein): Functionalized Mesoporous silica (FMS) Si CH2 CH2 CH2 SH Si CH2 CH2 CH2 NH2 Si CH2 CH2 COOH Schematic drawing of FMS. Feng, X.; Fryxell, G. E.; Wang, L. –Q.; Kim, A. Y.; Liu, J.; Kemner, K. M. Science 1997, 276, 923-926. OPH structural dimensions & amino acid residues 92 Å 40 Å 56 Å OPH structure with charged surface residues: lysine (red), arginine (green), glutamic acid (yellow) displayed by “ball and stick”. The majority of the protein is displayed by “backbone”. Covalently linking protein in FMS O Si CH2 CH2 CH2 O NH2 + H C CH2 CH2 CH2 C H O Si CH2 CH2 CH2 N CH CH2 CH2 CH2 C H O Si CH2 CH2 CH2 N CH CH2 CH2 CH2 C H + H2N Si CH2 CH2 CH2 N CH CH2 CH2 CH2 CH N Protein Protein Reaction of NH2-FMS with GDAH and subsequently with the enzyme. Spontaneously entrapping protein in FMS Quic kTime™ and a Sorenson Video 3 dec ompress or are needed to see this picture. Organophosphorous Hydrolase (OPH) Structure of Organophosphorus Compounds: Toxicities of Organophosphorus compounds: O CH3 (CH3)2CH O P F Soman O CH3CH2 O P SCH2CH2N CH3 CH(CH3)2 O CH2CH3 Paraoxon 150-600 Parathion 13 Paraoxon 0.5 Sarin 0.01 Soman 0.01 Tabun 0.01 VX 0.001 Palytoxin 0.00015 Botulinum toxin 0.000001 CH(CH3)2 VX O Diazinon CH3 Sarin CH3CH2 O P O Approx. LD50 (mg/kg, iv.) O (CH3)3C CH O P F CH3 Compounds NO2 O (CH3)2N P CN O CH2CH3 Tabun Comparison of different porous silica support for OPH immobilization 250 OPH Protein Amount 0.05 OPH Activity 0.04 200 150 0.03 100 0.02 50 0.01 0 5000 OPH Entrapped in HOOC-FMS OPH Specific Activity (Units/mg of protein) 2% N PS H 20 O U % O MS H C 2% O -NP O S 20 HO C-N % O P H C- S O FM 2 % OC S -F 20 NH MS % 2N N 2% H PS 220 NH NP % 2- S N FM H 2- S FM S 0 OPH Activity (units/mg of support) OPH Protein Amount (mg/mg of support) 0.06 4000 Free OPH in solution 3000 2000 1000 0 0 36 54 Time (days) Enhanced Specific Activity & Stability of Immobilized OPH 145 Biosensin, Filtation, Decontamination Paraoxon O CH3 CH2 O P O O CH2 CH3 NO2 OPH H2 O O CH3 CH2 O P OH + HO NO2 O CH2 CH3 p-nitrophenol Electrochemical Biosensing of Immobilized OPH in FMS to Organophosphorus 0.90x10-4 Buffer flush i /A Paraoxon addition 0.70x10-4 0.50x10-4 0.30x10-4 0 100 200 300 t/s At 0.90V. 400 500 What’s next? (1) We will integrate our extensive experiments with modeling/computation approaches To understand how enzyme stability and catalytic activity are enhanced; To better design nanomaterials; To screen the desired enzymes by genetic engineering; (2) We will try other enzymes of strategic significance, such as hydrogenase; (3) We will also try alternative nanomaterials, especially conductive one instead of silica; (4) Design and fabrication of biosensing devices and filtration/decontamination systems for Homeland Security, Army, and Environmental Protection.