* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Biologics for the respiratory physician - SGUL
Survey
Document related concepts
Transcript
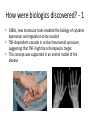
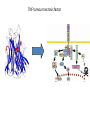
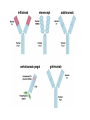
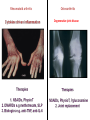
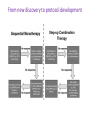
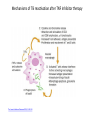
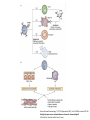
Biologics for the respiratory physician Nidhi Sofat Clinical Senior Lecturer St George’s, University of London What is a biologic? 1. Review of what they are/ their pharmacology/ classification 2. Brief overview of how and when used for rheumatological disease 3. The role of the resp physician - ruling out TB before use - protocol/ guidelines for doing this, evidence of TB - reactivation, immunology and science underlying this 4. Horizon scanning - are biologics ever likely to be useful for respiratory disease 1. Review of what they are/ their pharmacology/ classification How were biologics discovered? - 1 • 1980s, new molecular tools enabled the biology of cytokine expression and regulation to be studied • TNF-dependent cascade in active rheumatoid synovium, suggesting that TNF might be a therapeutic target • This concept was supported in an animal model of the disease Biology of TNF production, receptor interaction and signaling Tracey et al. Pharmacology & Therapeutics 117 (2008) 244–279 Tracey et al. Pharmacology & Therapeutics 117 (2008) 244–279 How were biologics discovered? - 2 • The proof of concept was a series of clinical trials, which have led to marked changes in the therapy of human rheumatoid arthritis and subsequently of other diseases • The work with TNF clearly demonstrated the importance of cytokines in medicine • Capacity of mAbs (or receptor fusion proteins) to be used long-term in large populations, thus changing the therapeutic landscape. TNF tumour necrosis factor Pharmacokinetics simulation of serum concentrations of infliximab, etanercept and adalimumab at steady state in patients with RA treated with each drug at the doses and schedules shown Tracey et al. Pharmacology & Therapeutics 117 (2008) 244–279 Tracey et al. Pharmacology & Therapeutics 117 (2008) 244–279 2. Brief overview of how and when used for rheumatological disease Rheumatoid arthritis Osteoarthritis Degenerative joint disease Burden of disease • RA affects ~1% of the population worldwide, was a major clinical problem. • With anti-cytokine therapy, RA is far from curable, but it is much more manageable, less crippling, and less lethal • RA is an autoimmune disease with chronic inflammation leading to joint destruction. • The classical autoantibodies, or rheumatoid factors, have long been known; more recently discovered are IgG Abs to deiminated citrullinated proteins. • Cytokines augment HLA expression and Ag presentation to T cells in local autoimmune disease sites like synovial joints • Cytokine research was propelled by the successful biochemical purification and cloning of cytokine cDNAs. • Molecular biology techniques also helped provide key therapeutics, such as engineered mAbs and receptor fusion proteins. Anti-TNF therapies Basic science data showed TNF may be key regulator in inflammation (Brennan et al. 1992) Infliximab, etanercept and adalimumab undergone extensive clinical trials for efficacy and safety Share common properties, but also have distinct pharmacologic and pharmacokinetic profiles Absence of direct comparison trials Clinicians need to compare efficacy First studies of TNF inhibitors • First clinical trial of infliximab in RA was in 1992, at Charing Cross Hospital (London, U.K.) • Open-label trial due to concerns about safety of a blinded clinical trial. Infliximab was well tolerated with no short-term safety signals and impressive efficacy, but as the Ab metabolized, all patients relapsed by 12– 18 wk • Next, a formal double-blind placebo-controlled randomized trial was conducted • A single infusion, with either a previously used effective dose (10 mg/kg) or a tenth of that, was used; both were accompanied by dramatic doserelated efficacy over 4 wk compared with placebo • From this trial, we were able to perform a series of studies on the mechanism of action of the anti-TNF Ab. ATTRACT study • 2 year, multicentre, multinational trial in 428 patients with active RA despite treatment with methotrexate • Randomized to one of 5 treatments • Infliximab 3 to 10 mg/mg every 4 to 8 weeks or placebo • Followed up over 2 years • All patients received stable dose of MTX mean 15mg/kg/week Maini et al, Lancet 1999 Main findings of ATTRACT • At 30 weeks, 50% met ACR50 criteria at infliximab 3mg/kg every 8 weeks, compared with 20% of placebo group • At 54 weeks, higher percentage of treatment group (3mg/kg) reached ACR20, ACR50 and ACR70 criteria compared with placebo group: • ACR 20 (42 VS 17%) P < 0.05 • ACR 50 (21 VS 8%) • ACR 70 (10 VS 2% ) • Effects sustained for up to 102 weeks Modified Sharp score and Infliximab (ATTRACT) Maini et al, Lancet 1999 Etanercept trials Genovese et al. 2001 Adalimumab study De Putte et al. 2003 Learning points • Within hours of anti-TNF administration, elevated levels of IL-6 in serum plummet virtually to baseline, thus confirming the existence of a TNFdependent cytokine network in RA patients in vivo • Rapid normalization of low hemoglobin, high platelet counts, and fibrinogen concentration and restoration of abnormal regulatory T cell function • Destructive enzymes (e.g., matrix metalloproteinase 3) are reduced, and osteoprotegerin levels are restored • Although there is a falloff of response to anti-TNF with time • Some patients have remained responsive to anti-TNF for over 5 yr. • This indicates that cytokine networks in RA evolve slowly, if at all, and if TNF is blocked, its pivotal role in orchestrating the inflammatory response is not displaced by another proinflammatory cytokine. From new discovery to protocol development Current TNF inhibitors in clinical practice • • • • • Adalimumab Certolizumab pegol Etanercept Golimumab Infliximab Drugs used to treat inflammatory arthritis Immunotherapy DMARDS Corticosteroids, Cyclophosphamide Anti-TNFα therapies Rituximab Anti-IL-6 tocilizumab Combination DMARDS Methotrexate, sulphasalazine, azathioprine NSAIDS and analgesics Traditional treatment Paradigm- Rheumatoid Arthritis Nice guideline Criteria common to many trials in RA • Effects on symptoms • ACR response criteria • Structure (joint space narrowing, erosions, Sharp scores) • Physical function/Quality of Life e.g. HAQ scores • WHY SO IMPORTANT • The total cost of RA in the UK has been estimated at between £3.8 and £4.75 billion per year. • 3. The role of the resp physician - ruling out TB before use - protocol/ guidelines for doing this, evidence of TB reactivation, immunology and science underlying this As Clinician – how do we treat? • Weigh evidence and experience with different agents • E.g. iv versus sc • Post-marketing surveillance • Risk factors e.g. TB • Long-term data: • BSR biologics register (10,000 patients) Why are trials important • They can change clinical practice • May allow comparisons between different treatments • May give rise to the discovery of new phenomena post-treatment e.g. incidence of TB post TNF therapy Models for attributing tuberculosis (TB) to drug treatment. Dixon W G et al. Ann Rheum Dis 2010;69:522-528 Cumulative incidence of tuberculosis (TB) following first exposure to anti-tumour necrosis factor (anti-TNF) therapy (most recent drug model, with person-years censored at death, last returned follow-up form, or date of switching to second anti-TNF). Dixon W G et al. Ann Rheum Dis 2010;69:522-528 Cumulative incidence of tuberculosis (TB) following first exposure to antitumour necrosis factor (anti-TNF) therapy • To April 2008, 40 cases of TB were reported, all in the anti-TNF cohort. • The rate of TB was higher for the monoclonal antibodies ADA (144 events/100 000 person-years) and INF (136/100 000 person-years) than for ETA (39/100 000 personyears). • After adjustment, the incidence rate ratio compared with ETA-treated patients was 3.1 (95% CI 1.0 to 9.5) for INF and 4.2 (1.4 to 12.4) for ADA. • The median time to event was lowest for INF (5.5 months) compared with ETA (13.4 months) and ADA (18.5 months). • 13/40 cases occurred after stopping treatment. 25/40 (62%) cases were extrapulmonary, of which 11 were disseminated. • Patients of non-white ethnicity had a sixfold increased risk of TB compared with white patients treated with anti-TNF therapy. Mechanisms of TB reactivation after TNF Inhibitor therapy Source: The Lancet Infectious Diseases 2003; 3:148-155 (DOI:10.1016/S1473-3099(03)00545-0) Interactions with infliximab and etanercept. Infliximab binds more avidly than etanercept does to transmembrane TNFα and forms a more stable complex More infliximab than etanercept binds to transmembrane TNFα. Infliximab is more effective at inhibiting transmembrane TNF -mediated activation of endothelial cells. Infliximab binds both the monomeric and trimeric form of soluble TNFα, whereas etanercept effectively binds only to the trimeric form. Etanercept–TNFα complexes are unstable, resulting in the release of soluble TNFα Source: The Lancet Infectious Diseases 2003; 3:148-155 (DOI:10.1016/S1473-3099(03)00545-0) • Thorax 2005;60:800-805 doi:10.1136/thx.2005.046797 • BTS guidelines • BTS recommendations for assessing risk and for managing Mycobacterium tuberculosis infection and disease in patients due to start anti-TNF-α treatment • British Thoracic Society Standards of Care Committee* • Correspondence to: Professor L P Ormerod Professor of Respiratory Medicine, Blackburn Royal Infirmary, Blackburn, Lancashire BB2 3LR, UK; [email protected] Algorithm for management of TB in patients scheduled for anti-TNF-α treatment. Copyright © BMJ Publishing Group Ltd & British Thoracic Society. All rights reserved. False-positive PPD test in a patient with psoriasis due to koebnerization Screening tests Characteristics Advantages/Disadvantages Suggested use Tuberculin Skin Test (TST) Not as sensitive or specific as QTF More widely available Requires a follow-up exam after placement within 48–72 h False positive with BCG vaccination Best tested before start of methotrexate or biologics More sensitive and specific than TST Best tested before the initiation of biologics Interferon gamma tests e.g. QTF / T spot Requires a blood draw and less widely available False positive with exposure to Mycobacterium marinum Possibility of pathergy to cause a false positive May lead to false negative during ongoing biologic therapy. Use in conjunction with exposure/travel history and symptomology Exposure and travel history Travel to endemic areas or exposure to known TB contacts elevate risk for Especially important after initiation of biologics as positive exposure or travel history should prompt a chest X-ray, even with negative TST or QFT Chest X ray Exposure to radiation Use in those with history concerning for possible TB exposure as TST and QTF are less reliable in ongoing biologic therapy Can follow internal lung changes in time Consider annual testing in those that are already on biologic therapy Mantoux versus Interferon gamma tests • • • • • Diagnosis of latent TB 1. Mantoux/TST testing should be performed 2. Interferon-gamma (IFN γ)– tests, if available, should be considered for those in whom Mantoux testing is positive or is less reliable. • • • • Type of tests Number of labs Mantoux test Quantiferon test T-spot test BTS recommendations for assessing risk and for managing Mycobacterium tuberculosis infection and disease in patients due to start anti-TNF-α treatment Table 3 Annual risk of TB disease/100 000 in England and Wales: effect of age (to the nearest whole number) Age (years) White Black African 0–14 1 47 15–34 2 314 35–54 4 168 55–74 7 204 >75 11 Not available •4. Horizon scanning - are biologics ever likely to be useful for respiratory disease Lessons learnt from biologic use in non-rheumatic diseases • The pharmacodynamics of most biologics is incompletely understood; for example, etanercept is ineffective in Crohn’s disease, whereas other antitumor necrosis factor (TNF) drugs are highly potent. • Induction of immune tolerance may be achievable in the early stages of autoimmunity • Biologic therapies have occasionally been associated with reactivation of latent viruses such as Epstein–Barr virus and JC virus. This may be inseparable from their mode of action, and highlights the need for careful post-marketing surveillance. • Many inflammatory diseases exist as several phenotypes, which respond differently to therapy. • As highlighted by asthma studies, it is essential to identify and consider these factors during clinical trial design in order to advance the field of stratified medicine, which is currently in its infancy. Nature Reviews Rheumatology 7, 507-516 (September 2011) | doi:10.1038/nrrheum.2011.106 Biologic therapies in non-rheumatic diseases: lessons for rheumatologists? Gillian M. Bell, Gary Reynolds & John D. Isaacs Summary • Monoclonal antibody technology has revolutionised rheumatology in the last 10 years • New therapeutic targets emerging in other specialties • Pathway development critical for optimal efficacy Acknowledgements • • • • • • • • • • • • • • • • People, St George’s Dr Alison Davis Ms Cori Smee Dr Monika Hermansson Dr Tom Barrick Dr Franklyn Howe Dr Julekha Wajed Dr Kaushik Sanyal Miss Saralili Dipa Robertson Mr Ray Moss Dr Matt Szarko Miss Caroline Hing Prof John Axford Prof Emma Baker Dr Patrick Kiely Dr Vivian Ejindu • • The Kennedy Institute of Rheumatology Dr Robin Wait • • Heatherwood and Wexham Park NHS Trust Mr Jonathan Jones Funding Rosetrees’ Trust St George’s Charitable Trust The Wellcome Trust South London AHSC NIHR CLRN