* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Respiratory syndrom
Ebola virus disease wikipedia , lookup
Oncolytic virus wikipedia , lookup
Introduction to viruses wikipedia , lookup
Plant virus wikipedia , lookup
Viral phylodynamics wikipedia , lookup
Virus quantification wikipedia , lookup
Social history of viruses wikipedia , lookup
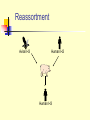
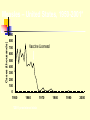
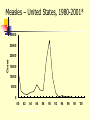
TASHKENT MEDICAL ACADEMY Infectious and children infectious diseases department Theme: Early and Comparative diagnosis of diseases with the respiratory syndrome Lecturer: Viruses Associated with Respiratory Infections Syndrome Commonly Associated Viruses Less Commonly Associated Viruses Corza Rhinoviruses, Coronaviruses Influenza and parainfluenza viruses, enteroviruses, adenoviruses Influenza Influenza viruses Parainfluenza viruses, adenoviruses Croup Parainfluenza viruses Influenza virus, RSV, adenoviruses Bronchiolitis RSV Influenza and parainfluenza viruses, adenoviruses Bronchopneumonia Influenza virus, RSV, Adenoviruses Parainfluenza viruses, measles, VZV, CMV Influenza Virus (Courtesy of Linda Stannard, University of Cape Town, S.A.) RNA virus, genome consists of 8 segments enveloped virus, with haemagglutinin and neuraminidase spikes 3 types: A, B, and C Type A undergoes antigenic shift and drift. Type B undergoes antigenic drift only and type C is relatively stable Influenza A Virus Undergoes antigenic shifts and antigenic drifts with the haemagglutinin and neuraminidase proteins. Antigenic shifts of the haemagglutinin results in pandemics. Antigenic drifts in the H and N proteins result in epidemics. Usually causes a mild febrile illness. Death may result from complications such as viral/bacterial pneumonia. Epidemiology Pandemics - influenza A pandemics arise when a virus with a new haemagglutinin subtype emerges as a result of antigenic shift. As a result, the population has no immunity against the new strain. Antigenic shifts had occurred 3 times in the 20th century. Epidemics - epidemics of influenza A and B arise through more minor antigenic drifts as a result of mutation. Past Antigenic Shifts 1918 H1N1 “Spanish Influenza” 20-40 million deaths 1957 H2N2 “Asian Flu” 1-2 million deaths 1968 H3N2 “Hong Kong Flu” 700,000 deaths 1977 H1N1 Re-emergence 2009 H1N1 “Swine Flu No pandemic Mild Pandemic At least 15 HA subtypes and 9 NA subtypes occur in nature. Up until 1997, only viruses of H1, H2, and H3 are known to infect and cause disease in humans. Avian Influenza H5N1 An outbreak of Avian Influenza H5N1 occurred in Hong Kong in 1997 where 18 persons were infected of which 6 died. The source of the virus was probably from infected chickens and the outbreak was eventually controlled by a mass slaughter of chickens in the territory. All strains of the infecting virus were totally avian in origin and there was no evidence of reassortment. However, the strains involved were highly virulent for their natural avian hosts. H9N2 Several cases of human infection with avian H9N2 virus occurred in Hong Kong and Southern China in 1999. The disease was mild and all patients made a complete recovery Again, there was no evidence of reassortment Theories Behind Antigenic Shift 1. Reassortment - Reassortment of the H and N genes between human and avian influenza viruses through a third host. There is good evidence that this occurred in the 1957 H2N2 and the 1968 H3N2 pandemics. The 2009 pandemic virus was thought to be novel virus that was a triple re-assortant involving human, bird, N. American pig and Eurasian pig viruses. 2. Recycling of pre-existing strains – this probably occurred in 1977 when H1N1 re-surfaced. 3. Gradual adaptation of avian influenza viruses to human transmission. There is some evidence that this occurred in the 1918 H1N1 pandemic. Reassortment Avian H3 Human H2 Human H3 Laboratory Diagnosis Rapid Diagnosis – nasopharyngeal aspirates, throat and nasal swabs are normally used. Antigen Detection – can be done by IFT or EIA RNA Detction – RT-PCR assays give the best sensitivity and specificity. It is the only method that can differentiate the 2009 pandemic H1N1 strain from the seasonal H1N1 strain. However, it is expensive and technically demanding. Virus Isolation - virus may be readily isolated from nasopharyngeal aspirates and throat swabs. Serology - a retrospective diagnosis may be made by serology. CFT most widely used. HAI and EIA may be used to give a type-specific diagnosis Management Neuraminidase inhibitors - are now the drugs. They are highly effective and have fewer side effects than amantidine. Oseltamivir (Tamiflu) is the most commonly used agent as it can be given orally unlike Zanamivir (Relenza). The resistance to different types varies enormously year to year. H3N2 strains were mainly sensitive whereas seasonal H1N1 were almost totally resistant. More than 98% of the 2009 pandemic influenza H1N1 tested were sensitive. Amantidine is effective against influenza A if given early in the illness. However, resistance to amantidine emerges rapidly. Rimantidine is similar to amantidine but but fewer neurological side effects. Ribavirin is thought to be effective against both influenza A and B. Prevention Inactivated split/subunit vaccines are available against influenza A and B. The vaccine is normally trivalent, consisting of one A H3N2 strain, one A H1N1 strain, and one B strain. The strains used are reviewed by the WHO each year. The vaccine should be given to debilitated and elderly individuals who are at risk of severe influenza infection. Amantidine can be used as an prophylaxis for those who are allergic to the vaccine or during the period before the vaccine takes effect. Parainfluenza Virus (Linda Stannard, University of Cape Town, S.A.) ssRNA virus enveloped, pleomorphic morphology 5 serotypes: 1, 2, 3, 4a and 4b No common group antigen Closely related to Mumps virus Clinical Manifestations Croup (laryngotraheobroncitis) - most common manifestation of parainfluenza virus infection. However other viruses may induce croup e.g. influenza and RSV. Other conditions that may be caused by parainfluenza viruses include Bronchiolitis, Pneumonia, Flu-like tracheobronchitis, and Corza-like illnesses. Laboratory Diagnosis Detection of Antigen - a rapid diagnosis can be made by the detection of parainfluenza antigen from nasopharyngeal aspirates and throat washings. Virus Isolation - virus may be readily isolated from nasopharyngeal aspirates and throat swabs. Serology - a retrospective diagnosis may be made by serology. CFT most widely used. Management No specific antiviral chemotherapy available. Severe cases of croup should be admitted to hospital and placed in oxygen tents. No vaccine is available. Respiratory Syncytial Virus (RSV) ssRNA eveloped virus. belong to the genus Pneumovirus of the Paramyxovirus family. Considerable strain variation exists, may be classified into subgroups A and B by monoclonal sera. Both subgroups circulate in the community at any one time. Causes a sizable epidemic each year. Clinical Manifestations Most common cause of severe lower respiratory tract disease in infants, responsible for 50-90% of Bronchiolitis and 5-40% of Bronchopneumonia Other manifestations include croup (10% of all cases). In older children and adults, the symptoms are much milder: it may cause a corza-like illness or bronchitis. Infants at Risk of Severe Infection 1. Infants with congenital heart disease - infants who were hospitalized within the first few days of life with congenital disease are particularly at risk. 2. Infants with underlying pulmonary disease - infants with underlying pulmonary disease, especially bronchopulmonary dysplasia, are at risk of developing prolonged infection with RSV. 3. Immunocompromized infants - children who are immunosuppressed or have a congenital immunodeficiency disease may develop lower respiratory tract disease at any age. Laboratory Diagnosis Detection of Antigen - a rapid diagnosis can be made by the detection of RSV antigen from nasopharyngeal aspirates. A rapid diagnosis is important because of the availability of therapy Virus Isolation - virus may be readily isolated from nasopharyngeal aspirates. However, this will take several days. Serology - a retrospective diagnosis may be made by serology. CFT most widely used. Treatment and Prevention Aerosolised ribavirin can be used for infants with severe infection, and for those at risk of severe disease. There is no vaccine available. RSV immunoglobulin can be used to protect infants at risk of severe RSV disease. Adenovirus (Linda Stannard, University of Cape Town, S.A.) ds DNA virus non-enveloped At least 47 serotypes are known classified into 6 subgenera: A to F Clinical Syndromes 1. Pharyngitis 1, 2, 3, 5, 7 2. Pharyngoconjunctival fever 3, 7 3. Acute respiratory disease of recruits 4, 7, 14, 21 4. Pneumonia 1, 2, 3, 7 5. Follicular conjunctivitis 3, 4, 11 6. Epidemic keratoconjunctivitis 8, 19, 37 7. Pertussis-like syndrome 5 8. Acute haemorrhaghic cystitis 11, 21 9. Acute infantile gastroenteritis 40, 41 10.Intussusception 1, 2, 5 11.Severe disease in AIDS and other immunocompromized patients 5, 34, 35 12. Meningitis 3, 7 Laboratory Diagnosis Detection of Antigen - a rapid diagnosis can be made by the detection of adenovirus antigen from nasopharyngeal aspirates and throat washings. Virus Isolation - virus may be readily isolated from nasopharyngeal aspirates, throat swabs, and faeces. Serology - a retrospective diagnosis may be made by serology. CFT most widely used. Treatment and Prevention There is no specific antiviral therapy. A vaccine is available against Adult Respiratory Distress Syndrome. It consists live adenovirus 4, 7, and 21 in enterically coated capsules. It is given to new recruits into various arm forces around the world. Common Cold Viruses Common colds account for one-third to one-half of all acute respiratory infections in humans. Rhinoviruses are responsible for 30-50% of common colds, coronaviruses 10-30%. The rest are due to adenoviruses, enteroviruses, RSV, influenza, and parainfluenza viruses, which may cause symptoms indistinguishable to those of rhinoviruses and coronaviruses. Rhinovirus Reconstructed Image of rhinovirus particle (Institute for Molecular Virology) ssRNA virus Belong to the picornavirus family ssRNA virus acid-labile at least 100 serotypes are known Coronavirus ssRNA Virus Enveloped, pleomorphic morphology 2 serogroups: OC43 and 229E Measles Highly contagious viral illness First described in 7th century Near universal infection of childhood in prevaccination era Frequent and often fatal in developing areas Measles Virus Paramyxovirus (RNA) One antigenic type Hemagglutinin important surface antigen Rapidly inactivated by heat and light Measles Pathogenesis Respiratory transmission of virus Replication in nasopharynx and regional lymph nodes Primary viremia 2-3 days after exposure Secondary viremia 5-7 days after exposure with spread to tissues Measles Clinical Features Incubation period 10-12 days Prodrome Stepwise increase in fever to 103 F or higher Cough, coryza, conjunctivitis Koplik spots Measles Clinical Features Rash 2-4 days after prodrome, 14 days after exposure Maculopapular, becomes confluent Begins on face and head Persists 5-6 days Fades in order of appearance Measles Complications Condition Diarrhea Otitis media Pneumonia Encephalitis Death Hospitalization Percent reported 8 7 6 0.1 0.2 18 Based on 1985-1992 surveillance data Measles Complications by Age Group 30 Pneumonia Hospitalization 25 Percent 20 15 10 5 0 <5 5-19 Age group (yrs) 20+ Measles Laboratory Isolation of measles virus fromDiagnosis a clinical specimen (e.g., nasopharynx, urine) Significant rise in measles IgG by any standard serologic assay (e.g., EIA, HA) Positive serologic test for measles IgM antibody Measles Epidemiology Reservoir Human Transmission Temporal pattern Peak in late winter and spring Communicability 4 days before to 4 days after rash onset Respiratory Airborne Measles – United States, 1950-2001* Cases (thousands) 900 800 Vaccine Licensed 700 600 500 400 300 200 100 0 1950 1960 *2001 provisional data 1970 1980 1990 2000 Measles – United States, 1980-2001* 30000 25000 Cases 20000 15000 10000 5000 0 80 82 84 86 *2001 provisional data 88 90 92 94 96 98 '00 Age Distribution of Reported Measles, 1975-2000 90 Preschool-age School-age Adult 80 Percent 70 60 50 40 30 20 10 0 1975 1980 1985 1990 Age group (yrs) 1995 2000 Measles Resurgence – United States, 1989-1991 Cases 55,622 Age group affected Children <5 yrs Hospitalizations >11,000 Deaths 123 Direct medical costs >$150 million Measles 1993-2001 Endemic transmission interrupted Record low annual total in 2000 (86 total cases) Many cases among adults Many cases due to importation Measles Clinical Case Definition Generalized rash lasting >3 days, and Temperature >38.3 C (101 F), and Cough, coryza, or conjunctivitis Measles Vaccines 1963 1965 1967 1968 1971 1989 Live attenuated and killed vaccines Live further attenuated vaccine Killed vaccine withdrawn Live further attenuated vaccine (Edmonston-Enders strain) Licensure of combined measlesmumps-rubella vaccine Two dose schedule Measles Vaccine Composition Live virus Efficacy 95% (range, 90%-98%) Duration of Immunity Schedule Lifelong 2 doses Should be administered with mumps and rubella as MMR MMR Vaccine Failure Measles, mumps, or rubella disease (or lack of immunity) in a previously vaccinated person 2%-5% of recipients do not respond to the first dose Caused by antibody, damaged vaccine, record errors Most persons with vaccine failure will respond to second dose Measles (MMR) Vaccine Indications All infants >12 months of age Susceptible adolescents and adults without documented evidence of immunity Measles Mumps Rubella Vaccine 12 months is the recommended and minimum age MMR given before 12 months should not be counted as a valid dose Revaccinate at >12 months of age Second Dose of Measles Vaccine Intended to produce measles immunity in persons who failed to respond to the first dose (primary vaccine failure) May boost antibody titers in some persons Second Dose Recommendation First dose of MMR at 12-15 months Second dose of MMR at 4-6 years Second dose may be given any time >4 weeks after the first dose ACIP Recommendations All states ensure that 2 doses of MMR required for school entry All children in kindergarten through grade 12 have 2 doses of MMR by 2001 Adults at Increased Risk of Measles College students International travelers Health-care personnel Measles Immunity in Health Care Personnel All persons who work in medical facilities should be immune to measles Measles Immunity Born before 1957 Documentation of physiciandiagnosed measles Serologic evidence of immunity Documentation of receipt of measles-containing vaccine Measles Vaccine Indications for Revaccination Vaccinated before the first birthday Vaccinated with killed measles vaccine Vaccinated prior to 1968 with an unknown type of vaccine Vaccinated with IG in addition to a further attenuated strain or vaccine of unknown type MMR Adverse Reactions Fever Rash Joint symptoms Thrombocytopenia Parotitis Deafness Encephalopathy 5%-15% 5% 25% <1/30,000 doses rare rare <1/1,000,000 doses MMR Vaccine and Autism Measles vaccine connection first suggested by British gastroenterologist Diagnosis of autism often made in second year of life Multiple studies have shown no association MMR Vaccine and Autism “The evidence favors a rejection of a causal relationship at the population level between MMR vaccine and autism spectrum disorders (ASD).” - Institute of Medicine, April 2001 MMR Vaccine Contraindications and Precautions Severe allergic reaction to prior dose or vaccine component Pregnancy Immunosuppression Moderate or severe acute illness Recent blood product Measles and Mumps Vaccines and Egg Allergy Measles and mumps viruses grown in chick embryo fibroblast culture Studies have demonstrated safety of MMR in egg allergic children Vaccinate without testing Measles Vaccine and HIV Infection MMR recommended for persons with asymptomatic and mildly symptomatic HIV infection NOT recommended for those with evidence of severe immuno- suppression Prevaccination HIV testing not recommended PPD and Measles Vaccine Apply PPD at same visit as MMR Delay PPD >4 weeks if MMR given first Apply PPD first - give MMR when skin test read