* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Molecular Hamiltonian wikipedia , lookup
Transition state theory wikipedia , lookup
Marcus theory wikipedia , lookup
Host–guest chemistry wikipedia , lookup
Metastable inner-shell molecular state wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Molecular orbital wikipedia , lookup
Chemical bond wikipedia , lookup
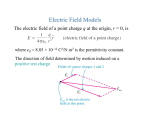
Heat transfer physics wikipedia , lookup
Rotational spectroscopy wikipedia , lookup
Atomic theory wikipedia , lookup
Rotational–vibrational spectroscopy wikipedia , lookup
2.2 Charge-Dipole Interaction Review (Isr2011, sec 4.1) What is a dipole? How are polar molecules formed? Order of magnitude of molecular dipole Charge-Dipole Interaction (Isr2011, sec 4.3) In isolation In medium 1 What Is Dipole? Dipole Two equal charges, q, of opposite sign, separated by a distance l, constitutes an electric dipole. A dipole u is represented as a vector pointing from –q to +q and has a magnitude of q l -q +q JCheng201209 u q 2 Two Types of Polar Molecules Polar molecules Molecules carrying no net charge but possessing an electric dipole Inherent polar molecules E.g. in HCl, Cl atom tends to draw the hydrogen’s electron toward itself (as indicated in the electron cloud around the nuclei of Cl and H), forming a permanent dipole (indicated as blue arrow) JCheng201209 http://en.wikipedia.org/wiki/HCl, http://en.wikipedia.org/wiki/Water, 3/3/2009 3 Two Types of Polar Molecules Environment-dependent polar molecules contd “The dipoles of some molecules depend on their environment and can change substantially when they are transferred from one medium to another, especially when molecules become ionized in a solvent.” (Isr2011, p. 71) E.g. glycine (amino acetic acid) in water becomes a dipolar molecule JCheng201209 4 Order of Magnitude of Molecular Dipoles Debye 1 Debye (1 D) = 3.336 x 10-30 C-m E.g. A dipole of two charges, e, separated by 1 Å = 4.8 D JCheng201209 5 Order of Magnitude of Molecular Dipoles contd JCheng201209 6 Order of Magnitude of Molecular Dipoles contd JCheng201209 7 Order of Magnitude of Molecular Dipoles contd Use bond moments to estimate molecular dipoles as shown in previous table E.g., using bond moment O-H to estimate the dipole of H2O Exercise. Try other molecular dipoles. CH3OH (methanol, 甲醇) CH3COOH (assuming COOH are on the same plane) CH3Cl 120o JCheng201209 Hint: 8 Order of Magnitude of Molecular Dipoles contd A case where simple addition of individual bond moments does not give right prediction Chloroform: CHCl3 JCheng201209 I guess the reason is the same one as that causing hydrogen bonding in chloroform “A hydrogen attached to carbon can also participate in hydrogen bonding when the carbon atom is bound to electronegative atoms, as is the case in chloroform, CHCl3” (from wikipedia http://en.wikipedia.org/wiki/Hydrogen_bonding, 20131022) 9 Charge-Dipole Interaction in Isolation +q Dipole u = ql Assume r >> l The interaction energy JCheng201209 r +Q -q Qu cos 1 Q r u w( r , ) 2 40 r 40 r 3 E u (derive it) 10 Charge-Dipole Interaction in Isolation contd Example Estimate max. interaction energy between a water molecule and Na+ Assumptions Water: a spherical molecule, r = 1.4 Å, u = 1.85 D Na+: r = 0.95 Å (r: radius) (Ans: 96 kJ mol-1 = 39 kBT at 300 K) * kB: Boltzmann constant = 1.38 x 10-23 J/K JCheng201209 11 Charge-Dipole Interaction in Medium r = + +Q randomly oriented r = finite +Q most likely to point around 0o Q. How to determine the intermolecular potential? If we know the probability distribution of , we can w(r ) w(r, ) r Q1. What is the probability distribution of ? JCheng201209 To appendix on Boltzmann distribution 12 Charge-Dipole Interaction in Medium contd z dir +Q Boltzmann distribution theorem predicts the probability density function p(,) using w(,r) Prob([ , d), [, d)) p(, ) dA Boltzmann dist.theorem C0 e where 1 C0 0 JCheng201209 2 0 e w ( r , ) k BT 2 w ( r , ) k BT C0 e w ( r , ) k BT dA 2 sin dd 2 sin dd i.e. 2 1 p(, ) dA 13 Charge-Dipole Interaction in Medium contd Average potential w(r) becomes w( r ) w( r, ) r 0 C0 0 2 0 2 0 w( r, ) e 2 w( r, ) p(, ) sin d d 2 w ( r , ) k BT 2 sin dd 2 For r >> 1, w(r) becomes r>>1 2 1 Qu 1 2 2 w ( r ) w ( r , ) r uE 2 3kT 40r 3kT 1 4 r * Note this equation for charge-dipole interaction is from Atkin’s textbook on Physical Chemistry, 7th ed. 2000. It is twice the value derived in Israelachvili 1991. JCheng201209 14 Interaction between Charge & Dipole in a Medium in Near Neighborhood Consider a charge +Q is placed right inside a fluid made of polar molecules Polar molecules distributed according to Boltzmann distribution What is free energy in this condition? The average free energy is still <w(r,)> Unfortunately, because |w(r,)| << kT is no longer valid, no simple formula as in previous case is possible. JCheng201209 15