* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Topics • Introduction • Molecular Structure and Bonding • Molecular
Ring-closing metathesis wikipedia , lookup
Jahn–Teller effect wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Metal carbonyl wikipedia , lookup
Metalloprotein wikipedia , lookup
Hydroformylation wikipedia , lookup
Stille reaction wikipedia , lookup
Spin crossover wikipedia , lookup
Topics
•
•
•
•
•
•
•
Introduction
Molecular Structure and Bonding
Molecular Orbital Theory
Molecular Symmetry
Coordination Complexes
Reactions of Metal Complexes
Organometallic Chemistry
Housecroft 6.11-6.13, 20.9-20.11, 25
Shriver and Atkins Chapter 7,14
Reactions of Metal Complexes
• Formation constants
– the chelate effect
– Irving William Series
– Lability
• Reaction Mechanisms
– I, A, D Mechanisms
– a, d Rate Determining Step
• Substitution of Square Planar Complexes
– the trans effect
• Substitution of Octahedral Complexes
1
Formation of Coordination Complexes
• typically coordination compounds are more
labile or fluxional than other molecules
MX + Y
MY + X
• X is leaving group and Y is entering group
• One example is the competition of a ligand, L
for a coordination site with a solvent molecule
such as H2O
[Co(OH2)6]2+ + Cl-
[Co(OH2)5Cl]+ + H2O
Formation Constants
• Consider formation as a series of formation
equilibria:
M+L
ML
ML2
ML + L
K1 =
[ ML]
[ M ][ L]
K2 =
[ ML2 ]
[ ML][ L]
• Summarized as:
M + nL
MLn
βn =
[ MLn ]
= K1 K 2 K 3 ...K n
[ M ][ L]n
2
Values of Kn
• Typically:
Kn-1>Kn
– Expected statistically, fewer coordination sites
available to form MLn than MLn-1
– eg sequential formation of [Al(OH2)6-x(F)x](3-x)+
Breaking the Rules
• Order is reversed when some electronic or
chemical change drives formation
Fe(bipy)2(OH2)22+ + bipy
Fe(bipy)32+
– jump from a high spin to low spin complex
• Fe(bipy)2(OH2)2 t2g4eg2 high spin
• Fe(bipy)3
t2g6
low spin
N
N
2,2'-bipyridine = bipy
3
Chelate Effect
• Compare: K1 to β2 for:
M(OH2)22+ + en
M(OH2)22+ + 2NH3
M(en)2+ + 2H2O
M(NH3)22+ + 2H2O
• Basically equivalent chemistry but for Cu2+
log K1 =10.6
log β2 =7.7
• chelated complex is three orders of magnitude more
stable
• chelate effect: the enhanced stability of a chelated
complex over its non-chelating analog
• attributed to the change in entropy, chelation trades
two restricted solvent molecules for one bound ligand
Ring Formation and Electron Delocalization
• Ability to form rings with metal center
improves stability
– particularly five or six membered rings
• Additionally, ligands with aromatic rings can
behave as pi acceptors and form back
bonding complexes
N
N
N
Ru
N
N
N
4
Irving William Series
• Values of log Kf for 2+
ions including transition
metal species
• Kf series for transition
metals:
Mn2+< Fe2+< Co2+< Ni2+< Cu2+>Zn2+
Irving Williams Series
• Partially explained by electrostatics: smaller
metal centre, same charge = greater charge
density
• Based on electrostatics we expect stabilities
which vary as:
Mn2+< Fe2+< Co2+< Ni2+ > Cu2+>Zn2+
• Irving William Series gives Cu2+ more stable
than Ni2+
– Because of Jahn Teller Distortion
5
Ni2+ vs Cu2+ Kf
• Stepwise Kf for displacement of H2O by
NH3 ligands from aquated Ni2+ and Cu2+
Reaction Mechanisms of d Metal Complexes
• We’ve been considering the equilibrium
formation
• Rate is important for understanding
coordination complex chemistry
– Inert: species that are unstable but survive for
minutes or more
– Labile: species that react more rapidly than inert
complexes
6
Labile vs. Inert
• General Rules:
– For 2+ ion, d metals are moderately labile
particularly d10 (Hg2+, Zn2+)
– Strong field d3 and d6 octahedral complexes are
inert .i.e. Cr(III) and Co(III)
– Increasing Ligand Field Stabilization Energy
improves inertness
– 2nd and 3rd row metals are generally more inert
Ligand Field Stabilization Energy (LFSE)
• Consider the energy of the d orbitals before crystal
field splitting relative to the first three possible
electronic configurations
7
LFSE for Oh Geometry
dn
d1
d2
d3
d4
d5
High Spin
config
t2g1eg0
t2g2eg0
t2g3eg0
t2g3eg1
t2g3eg2
FSE (∆o)
-0.4
-0.8
-1.2
-0.6
0
d6
d7
d8
d9
d10
t2g4eg2
t2g5eg2
t2g6eg2
t2g6eg3
t2g6eg4
-0.4
-0.8
-1.2
-0.6
0
Low Spin
config
FSE(∆o)
t2g4eg0
t2g5eg0
-1.6
-2.0
t2g6eg0
t2g6eg1
-2.4
-1.8
LFSE: e- configuration determines stabilization
8
Associative vs Dissociative Reactions
• Ligand substitution reactions are either
associative or dissociative
– Associative: reaction intermediate has higher
coordination number than reactants or products
• lower coordination number complexes
• Rates depend on the entering group
– Dissociative: reaction intermediate has lower
coordination number than reactants or products
• Octahedral complexes and smaller metal
centers
• Rates depend on leaving group
Patterns of Reactivity
• Formation constants tell us about
thermodynamics
• Kinetics requires a different measure:
nucleophilicity
– Ligand displacement are nucleophilic substitution
reactions
– The rate of attack on a complex by a given ligand
(Lewis Base) relative to the rate of attack by a
reference base.
• Rates span from 1 ms to 108 s
9
Ligand Labels for Nucleophilic Substitutions
• Three types of ligands can be important:
– Entering Ligand: Y
– Leaving Ligand: X
– Spectator Ligand
• Species that neither enters nor leaves
• Particularly important when located in a Trans
position, designated T
Reaction Mechanisms
• Associative - A (2 steps)
MLnX + Y
MLnXY
MLnY + X
• Dissociative - D (2 steps)
MLnX + Y
MLn + X + Y
MLnY + X
• Interchange (1 continuous process)
MLnX + Y
Y--MLn --X
MLnY + X
10
Rate Determining Step
• also denoted associative or dissociative
• associative (lowercase a)
– the rate depends heavily on the entering group
[PtCl(dien)]+ + I[PtCl(dien)]+ + Br-
[PtI(dien)]+ + Cl[PtBr(dien)]+ + Cl-
• dissociative (lowercase d)
– the rate is independent of the entering group
[Ni(OH2)6]2+ + NH3
[Ni(OH2)5(NH3)]2+ + H2O
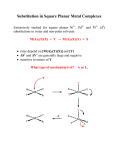
Substitution of Square Planar Complexes
• substitution of square planar complexes is
almost always Aa mechanisms
– rate depends on the entering group
– rate determining step is the M-Y bond formation
• impacted by the Trans effect
– the ligand trans to the leaving ligand (X) can alter
the reaction rate
11
Square Planar Substitution: The Trans Effect
• when the ligand, T, trans to the leaving group
in square planar complexes effects the rate of
substitution
• If T is a strong σ donor or π acceptor, the rate
of substitution is dramatically increased
• why?
– if T contributes a lot of e- density (is a good σ
donor) the metal has less ability to accept electron
density from X (the leaving ligand)
– if T is a good π acceptor, e- density on the metal is
decreased and nucleophilic attack by Y is
encouraged
Trans Effect Strengths
• Trans effect is more pronounced for σ donor
as follows:
OH-<NH3<Cl-<Br-<CN-,CO, CH3-<I-<PR3
• Trans effect is more pronounced for a π
acceptor as follows:
Br-<Cl-<NCS-<NO2-<CN-<CO
12
Using the Trans Effect
• Suggest a means to synthesize cis and trans
[PtCl2(NH3)2] from [Pt(NH3)4]2+ and [PtCl4]2-
Square Planar Substitution: Steric Effects
• steric crowding reduces the rate of A
mechanisms and increases D mechanisms
• simply a spatial phenomenon:
– less room around the metal means that a higher
coordination number transition state is higher
energy
• eg cis-[PtXL(PEt3)2]
• rate varies with L
• pyridine > 2-methyl py >
2,6-dimetyl py
13
Square Planar Substitution: Stereochemistry
• observing the final product stereochemistry
can provide information on the mechanism
and intermediate lifetimes
Square Planar Substitution: Volume of
Activation
• changes in volume along a reaction pathway
can be determined
• usually by observing reaction rate as a
function of pressure
• a negative ∆V‡ suggests an associative
complex
14
Square Planar Substitution: Entropy of
Activation
• the change in entropy from the reactants to
the activated complex is ∆S‡
• determined by the temperature dependence
of the rate
• associative mechanism has –’ve ∆S‡
• as expected from increasing order of the
system by loss of freedom for the entering
group without release of the leaving group
Substitution of Square Planar Complexes
• Trans Effect – ligand trans to X can increase
substitution if it is a good σ donor or π acceptor
• Steric Effects – bulky cis ligands reduce Y
nucleophilic attack
• Stereochemistry – cis/trans conserved for A
mechanism unless activated complex is long
lived
• ∆V‡ and ∆S‡ are both negative for A mechanism
15
Substitution of Octahedral Complexes
• I is the most important reaction mechanism
for substitution of Oh complexes
• but is it Ia or Id
– recall it depends on the rate determining step
being Y—M formation vs M—X breaking
– associative (lowercase a)
• the rate depends heavily on the entering group
– dissociative (lowercase d)
• the rate is independent of the entering group
Eigen-Wilkins Mechanism
• The standard mechanism for Oh I
substitutions reactions
• Based on the formation of an “encounter
complex”
• Fast pre-equilibrium:
ML6 + Y
{ML6,Y}
KE =
[{ML6 , Y }]
[ ML6 ][Y ]
• Followed by product formation:
{ML6,Y}
product
rate = k[{ML6 , Y }]
16
Eigen-Wilkins Mechanism II
• The rate expression can be written in terms of
the KE so that:
rate =
kK E [C ]tot [Y ]
1 + K E [Y ]
• Where [C]tot is the total of all of the complex
species
• If KE[Y] << 1 then the rate becomes:
rate = kobs [C ]tot [Y ]
Using Eigen Wilkins
• kobs = kKE so we can get k
• Now test k to see if it varies with Y or not so
we can assign Ia or Id
• Whew!
• See table 14.6 for experimental data
17
Oh Substitution General Rules
• Most 3d metals undergo Id substitutions
– I.e. the rate determining step is independent of the
entering group and primarily is the breaking of the
M—X bond
• Larger metals (4d, 5d) lean towards Ia
• Also low d electron density encourages partly
Ia characteristics
Oh: Effects of Ligands
• Leaving Group
– Nature of X is important as expected for Id as bond
breaking of M-X is the rate determining step
• Spectator ligands (cis-trans effect)
– No clear trans effect for Oh complexes
– In general, good spectator sigma donors will
stabilize the complex after the departure of the
leaving group
18
Oh: Steric Effects on Substitution
• steric crowding around the metal centre
favors dissociative activation
• Dissociative activation relieves crowding
around the complex
• Steric crowding has been qualitatively and
quantitatively explored
– Tolman Cone Angle
– See Table 14.7
Octahedal Substitution and ∆V‡
M(OH2)6 + H217O
• For I mechanism,
∆V‡ is not large but
Ia tends to be –’ve,
Id tends to be +’ve
• decreasing d
number shows
tendancy towards Ia
mechanism
M(OH2)5(17OH2) + H2O
M2+
Ti2+
V2+
Cr2+
Mn2+
Fe2+
Co2+
Ni2+
d elec.
2
3
4
5
6
7
8
∆V‡
-4.1
-5.4
+3.8
+6.1
+7.2
19
Oh Stereochemistry of Substitution
•
•
•
•
More complicated than for Td complexes
Example: cis- or trans- [CoAX(en)2]2+
cis complexes tends to retain cis
trans complexes can isomerize depending on
the spectator ligand, depends on geometry of
the activated complex
– Trigonal bipyramidal results in isomerization
depending on where Y enters
– Square planar leads to retention of
stereochemistry
20
Isomerization Reactions
• Similar to substitution reactions
• Berry Pseudorotation mixes axial and equatorial
positions in a 5 coord TBP species
• Both square planar complexes which undergo A
mechanisms or Oh complexes which undergo D or Id
mechanisms involve a 5 coordinate state so …
isomerization is possible
Twisted Oh Isomerizations
• Oh complexes may also isomerize via “twist”
mechanisms
• Does not require loss of ligands or breaking
bonds, just depends on energy barriers
between confirmations
– Bailar Twist (a)
– Ray Dutt Twist (b)
• Both occur via trigonal prismatic
confirmation
21
Twists
Redox Reactions
• Requires transfer of electrons in form of
straight electrons
– Like electrochemical cell, transfer from one metal
to another
– Transfer of group of ligands along with their
electrons to effectively reduce or oxidize a metal
centre
– Shriver and Atkins: Chapter 14
– Housecroft and Sharpe: Chapter 25
22
Redox Reactions
• Two reaction mechanisms
– Inner sphere
• Requires formation of bridged bimetallic
species
• results in ligand transfer at the same time
– Outer sphere
• No bridging ligand involved
• Direct transfer of electrons between the metal
centres
Outer Sphere Reaction Mechanisms
• Readily identified when no ligand transfer
occurs between the species
• Easier to identify when complexes are inert
with respect to ligand substitution
• Born Oppenheimer Approximation
– Electrons move faster than nuclei
– Complexes reorganization can be considered in a
separate step from electron transfer
• Marcus Equation
– Electron transfer requires vibrational excited
states, shape of potential energy well determines
rate of transfer
23
Inner Sphere Reactions
• Require the presence of bridging ligands
– Ligands with multiple pairs of electrons to donate
Cl-
S
C N-
N N
C N-
• Rate of electron transfer is dependent on the
ligands that are present
• See table 14.11 in Shriver and Atkins or table
25.8 in Housecroft and Sharpe
Inner Sphere Reaction Steps
• Formation of Bridged Complex
MIIL6
+
XMIIIL5'
L5MII XMIIIL5'
+
L
• Electron Transfer
L5MII XMIIIL5'
L5MIII XMIIL5'
• Decomposition into Final Products
L5MIII XMIIL5'
products L5MIII X
+
MIIL5'
24
Rate Determining Step
• Usually the electron transfer step
• However formation of bridging complex or the
decomposition could also limit the rate
• Where rds is electron transfer
– Good conjugation could provide a simple path for
the electron
• Studied via construction of bridging ligand
systems as models
Conclusions
• Reaction mechanisms
– A basic description of different mechanisms for
• Ligand exchange
• Isomerization
• Electron transfer
– Emphasis on ligand substitution reactions
• Determination of I, A, D mechanisms
• a vs d activation
25