* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Reaction mechanism of Coordination Complexes
Ring-closing metathesis wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Metal carbonyl wikipedia , lookup
Metalloprotein wikipedia , lookup
Hydroformylation wikipedia , lookup
Jahn–Teller effect wikipedia , lookup
Spin crossover wikipedia , lookup
Stille reaction wikipedia , lookup
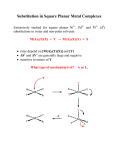
Reaction mechanism of Coordination Complexes Complexes are classified as Inert and Labile ( kinetic stability) depending on their reactivity. According to Henry Taube, a Nobel Laureate, the definition is If the half life period of 0.1 M solution at RT is less than 1 min. ( t½ < 1 min) the complex is called Labile If the half life period of 0.1 M solution at RT is more than 1 min. ( t½ > 1 min) the complex is called Inert SUBSTITUTION REACTIONS IN COMPLEXES Complexes usually undergo substitution reactions by the replacement of ligands with others. The reaction is common in Octahedral and Square planar complexes and follows two different paths SN1 and SN2 I.Dissociation Mechanism (SN1 ) Step (i) Slow step : dissociation of ligand (Y) MX5Y ( CN = 6 ) Octahedral srrchemistrylessons MX5 + Y ( CN = 5 ) Trigonal bipyramidal 1 Step (ii) Fast step : Addition of new ligand (Z) MX5 ( CN = 5 ) Trigonal bipyramidal MX5 Z ( CN = 6 ) Octahedral Mechanism : srrchemistrylessons 2 II. Association Mechanism (SN2 ) Step (i) Slow step : Association of ligand (Z) to complex MX5Y + Z ( CN = 6 ) Octahedral Step (ii) MX5 YZ ( CN = 7 ) Pentagonal bipyramidal Fast step : Leaving the ligand (Y) MX5 YZ ( CN = 7 ) Pentagonal bipyramidal srrchemistrylessons MX5 Z ( CN = 6 ) Octahedral 3 Substitution reactions in Square Planar complexes Association mechanism is favorable in square planar complexes Association Mechanism (SN2 ) Step (i) Slow step : Association of ligand (Z) to complex MX3Y + Z ( CN = 4 ) Square planar Step (ii) MX3 YZ ( CN = 5 ) Trigonal bipyramidal Fast step : Leaving the ligand (Y) MX3 YZ ( CN = 5 ) Trigonal bipyramidal srrchemistrylessons MX3 Z ( CN = 4 ) Square planar 4 Trigonal bipyramidal srrchemistrylessons 5 TRANS EFFECT It is observed that during the substitution reactions of square planar metal complexes, some ligands preferentially direct the substitution trans to themselves. i.e., the choice of leaving group is determined by the nature of ligand trans to it. The Trans effect can be defined as the effect of a ligand over rate of substitution of another ligand positioned trans to it in the square planar complexes. The ability of a ligand to direct the incoming group to the position trans to it, is called trans directing effect The rough order of trans-directing effect of various ligands is: NO+, CO, CN-, C2H4 > PR3, H- > Me- > Ph- > NO2-, I-, SCN- > Br- > Cl- > Py, NH3, OH-, H2O Ex 1: In the second step, the trans-directing effect of therefore cis-isomer is formed Cl- > NH3 In the second step, the trans-directing effect of therefore trans-isomer is formed Cl- > NH3 Ex 2: srrchemistrylessons 6 Explanation : Ex 3: synthesis of cis and trans – [Pt (NH3)(NO2)Cl2] srrchemistrylessons 7