* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download hap2 - WordPress.com
Survey
Document related concepts
Biochemical cascade wikipedia , lookup
Cell culture wikipedia , lookup
Artificial cell wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Animal nutrition wikipedia , lookup
Symbiogenesis wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Developmental biology wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Cell (biology) wikipedia , lookup
Signal transduction wikipedia , lookup
Cell theory wikipedia , lookup
Transcript
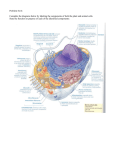
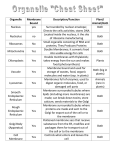
Lecture 2 Chemical Basis of Life Introduction: A. Chemistry deals with the composition of substances and how they change. B. A knowledge of chemistry is necessary for the understanding of physiology because of the importance of chemicals in body processes. Structure of Matter: A. Elements and Atoms: 1. Matter is anything that takes up space. 2. All matter is composed of elements, 92 of which occur naturally. 3. Living organisms require about 20 elements, of which oxygen, carbon, hydrogen, and nitrogen, phosphorus and sulfure are most abundant. If Ca included (99%) 4. Elements are composed of atoms; atoms of different elements vary in size and in how they interact. C. Bonding of Atoms: 1. Ionic bond 2. Covalent bond 3. Disulfide bond 4. Hydrogen bond C. Bonding of Atoms: 1. Atoms form bonds by gaining, losing, or sharing electrons. 2. Electrons are found in shells around the nucleus. a. The first energy shell holds two electrons; the other energy shells each hold eight electrons when on the outside. C. Bonding of Atoms: 3. Atoms with incompletely filled outer shells tend to be reactive to form stable outer shells of 8. 4. When atoms gain or lose electrons, they become ions with a charge. Whether they gain or lose will depend on how many they have in the outer shell to start with. 5. Oppositely-charged ions attract each other and form an ionic bond. E. Formulas: 1. A molecular formula represents the numbers and types of atoms in a molecule. 2. Various representations, called structural formulas, can be used to illustrate molecules. G. Acids and Bases: 1. Substances that release ions in water are called electrolytes. 2. Electrolytes that release hydrogen ions in water are called acids. 3. Electrolytes that release ions that combine with hydrogen ions in water are called bases. G. Acids and Bases: 4.The concentrations of H+ & OH- in the body is very important to physiology. 5.pH represents the concentration of hydrogen ions [H+] in solution. Chemical Constituents of Cells: A. Compounds that contain both hydrogen and carbon are called organic, the others are inorganic 1. Water B. Inorganic Substances a. Water is the most abundant compound in living things and makes up two-thirds (60-75%) of the human body. b. Water is an important solvent so most metabolic reactions occur in water. c. Lubricant d. Temperature regulator B. 1. 2. Water Compartments Intracellular Fluid (65% of total body water) Extracellular fluid (35%) a.Plasma b.Lymph c. Tissue fluid or interstitial fluid d.Specializied fluid (Synovial fluid and Cerebrospinal fluid and aqueos humor) B. Inorganic Substances 2. Oxygen a.Oxygen is needed to release energy from nutrients and is used to drive the cell's metabolism. 3. Carbon Dioxide a.Carbon dioxide is released as a waste product during energy-releasing metabolic reactions. Energy is released in the form of ATP Blood gases (arterial blood tells about Respiratory and circulatory system) Nitric Oxide (NO) Endothelium of blood vessels Vasodilation Impulse transmission Immune system Clinical trials in Pulmonary H Premature birth babies C. Organic Substances: 1. Carbohydrates a. Carbohydrates provide energy for cellular activities and are composed of carbon, hydrogen, and oxygen. b. Carbohydrates are made from monosaccharides (simple sugars); disaccharides are two monosaccharides joined together; complex carbohydrates (polysaccharides), such as starch, are built of many sugars. Glucose+Glucose= Maltose Glucose+Fructose=Sucrose Glucose+Galactose=Maltose Starches-plant, cell-enzymes convert to ATP e.g Glycogen 2. Lipids: a. Lipids are insoluble in water and include fats, phospholipids, and steroids. b. Fats supply energy, are composed of oxygen, carbon, and hydrogen, and are built from glycerol and three fatty acids. 2. Lipids: c. Phospholipids contain glycerol, two fatty acids, and a phosphate group, and are important in cell structures. e.g Cell membrane, myeline d. Steroids are complex ring structures, and include cholesterol, which is used to synthesize the sex hormones. e. f. Chylomicrons by small intestine and lipoproteins by liver LDL and HDL CopyrightThe McGraw-Hill Companies, Inc. Permission required for reproduction or display. 3. Proteins: a. Proteins have a great variety of functions in the body---as structural materials, as energy sources, as certain hormones, as receptors on cell membranes, as antibodies, and as enzymes to catalyze metabolic reactions. 3. Proteins: b. Proteins contain C, O, H, and nitrogen atoms; some also contain sulfur. c. Building blocks of proteins are the amino acids, each of which has a carboxyl group, an amino group and a side chain called the R group. 3. Proteins: d. Proteins have complex shapes held together by hydrogen bonds. e. Protein shapes, which determine how proteins function, can be altered (denatured) by pH, temperature, radiation, or chemicals. 4. Nucleic Acids: a. Nucleic acids form genes and take part protein synthesis. b. They contain carbon, hydrogen, oxygen, nitrogen, and phosphorus, which are bound into building blocks called nucleotides. 4. Nucleic Acids: c. Nucleic acids are of two major types: DNA (with deoxyribose) and RNA (with ribose). d. RNA (ribonucleic acid) functions in protein synthesis; DNA (deoxyribonucleic acid) stores the molecular code in genes. Summary Chapter 3 Cells Introduction: A. The human body consists of 75 trillion cells that vary considerably in shape and size yet have much in common. B. 200 different kind of human cells B.Differences in cell shape make different functions possible. A Composite Cell: A. A composite cell includes many different cell structures. B.A cell consists of three main parts---the nucleus, the cytoplasm, and the cell membrane. RBC is exception C. Within the cytoplasm are specialized organelles that perform specific functions for the cell. D. Cell Membrane: 1. The cell membrane regulates the movement of substances in and out of the cell, participates in signal transduction, and helps cells adhere to other cells. 2. It is made of Phospholipids, cholesterol, proteins D. Cell Membrane: 2. General Characteristics a. The cell membrane is extremely thin and selectively permeable. b. It has a complex surface with adaptations to increase surface area. D. Cell Membrane: 3. Cell Membrane Structure: a. The basic framework of the cell membrane consists of a double layer of phospholipids, with fatty acid tails turned inward. b. Molecules that are soluble in lipids (gases, steroid hormones) can pass through the lipid bilayer. D. Cell Membrane: c. Embedded cholesterol molecules strengthen the membrane and help make the membrane less permeable to water-soluble substances. d. Many types of proteins are found in the cell membrane, including transmembrane proteins and peripheral membrane proteins. D. Cell Membrane: e. Membrane proteins perform a variety of functions and vary in shape. f. Some proteins function as receptors on the cell surface, starting signal transduction. g. Other proteins aid the passage of molecules and ions. D. Cell Membrane: h. Proteins protruding into the cell anchor supportive rods and tubules. i. Still other proteins have carbohydrates attached; these complexes are used in cell identification. Membrane proteins called cellular adhesion molecules (CAMs) help determine one cell’s interactions with others. Movements Through Cell Membranes A. The cell membrane controls what passes through it. B. Mechanisms of movement across the membrane may be passive, requiring no energy from the cell (diffusion, facilitated diffusion, osmosis, and filtration) or active mechanisms, requiring cellular energy (active transport, endocytosis, and exocytosis). Movements Through Cell Membranes (cont.) C. Passive Mechanisms 1. Diffusion a. Diffusion is caused by the random motion of molecules and involves the movement of molecules from an area of greater concentration to one of lesser concentration until equilibrium is reached. Movements Through Cell Membranes (cont.) C1b. Diffusion enables oxygen and carbon dioxide molecules to be exchanged between the air and the blood in the lungs, and between blood and tissue cells. Movements Through Cell Membranes C2.Facilitated Diffusion a. Facilitated diffusion uses membrane proteins that function as carriers to move molecules (such as glucose) across the cell membrane. b. The number of carrier molecules in the cell membrane limits the rate of this process. Movements Through Cell Membranes C3.Osmosis a. Osmosis is a special case of diffusion in which water moves from an area of greater water concentration (where there is less osmotic pressure) across a selectively permeable membrane to an area of lower water concentration (where there is greater osmotic pressure). Movements Through Cell Membranes A solution with the same osmotic pressure as body fluids is called isotonic; one with higher osmotic pressure than body fluids is hypertonic; one with lower osmotic pressure is hypotonic. Movements Through Cell Membranes C4. Filtration a. Because of hydrostatic pressure, molecules can be forced through membranes by the process of filtration. Blood pressure is a type of hydrostatic pressure. Movements Through Cell Membranes D. Active Mechanisms 1. Active Transport a. Active transport uses ATP to move molecules from areas of low concentration to areas of high concentration through carrier molecules in cell membranes. b. As much as 40% of a cell's energy supply may be used to fuel this process. Meiosis G. Cell aging Cell differentiation reflects genetic control of the nucleus as certain genes are turned on while others are turned off.