* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Patho Ch12
Cardiovascular disease wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Remote ischemic conditioning wikipedia , lookup
Heart failure wikipedia , lookup
Electrocardiography wikipedia , lookup
Drug-eluting stent wikipedia , lookup
Antihypertensive drug wikipedia , lookup
History of invasive and interventional cardiology wikipedia , lookup
Aortic stenosis wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Cardiac surgery wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Atrial septal defect wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Coronary artery disease wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
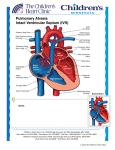
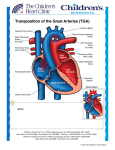
Pathology Ch12 (part) pp531-500 The Heart Congenital Heart Disease o Most CHD form during 3-8 week gestation o Incidence up to 5%, 85% composed of the following: Most common are VSD (42%) > ASD (10%) > Pulmonary stenosis (8%) > Patent ductus arteriosus > Tetralogy of Fallot > Coarcation of the aorta > AV septal defects > Aortic stenosis > Transposition of the great arteries > Truncus arteriosus Surgical repair successful unless irreversible changes have occurred o Cardiac Development Orchestrated by Wnt, SHH, VEGF, bone morphogenetic factor, TGFβ, FGF, Notch pathways Many inherited defects involve genes for transcription factors Mesoderm migrates to form first and second heart field @ day 15 Both heart fields are multipotent, but express different transcription factors First field (Hand1) >> L ventricle Second field (Hand2 + FGF10) >> R ventricle, most of atria, outflow tracts Beating tube formed @ day 20 Looping forms basic heart chambers @ day 28 Neural crest cells migrate into outflow tract > separate outflow and form aortic arches Interstitial CT (future AV canal and outflow) enlarges to produce endocardial cushions Further separation of ventricles, atria, AV valves produce 4-chamber heart @ day 50 o Etiology and Pathogenesis Sporadic genetic abnormalities are major known cause of CHD Heterozygous nature of mutation indicates that 50% reduction in function is enough to alter phenotype Many transcription factors interact in large protein complexes > any one defect can give similar results Ex. GATA4, TBX4, or NKX2-5 > ASD/VSD Genes that encode for various pathway components Ex. NOTCH1 > bicuspid aortic valve; NOTCH2 > Tetralogy of Fallot Ex. Fibrillin (Marfan's) mutation > lower than normal TGFβ signaling > Marfan heart anomalies Chromosomal lesions Ex. deletion of 22q11.2 (TBX1 deletion) > DiGeorge syndrome (CATCH-22) o Cardiac abnormality o Abnormal facies o Thymic aplasia o Cleft palate o Hypocalcemia Chromosomal aneuploidies Ex. Turner (45X), trisomies 13, 18, 21** (40% with DS have heart defects) Environmental factors alone or along with genetic factors Ex. Rubella infection, gestational diabetes, teratogen exposure, nutrition (folate) o Clinical Features Left-to-Right Shunts Initially NOT associated with Cyanosis High pulmonary vascular resistance can reverse the shunt (R>L) and introduce poorly oxygenated blood into systemic circulation [Eisenmenger syndrome] Atrial Septal Defect (ASD) o Usually asymptomatic until adulthood o Summary of developmental stages: Septum primum grows between R and L atria (opening "ostium primum") Ostium secundum develops before ostium primum is completely grown over Septum secundum grows to the R of septum primum (opening "foramen ovale" - continuous with ostium secundum, permit intrauterine shunting of blood) Septum secundum grows to form a flap of tissue over foramen ovale Flap of tissue permits shunting in fetal blood (higher R atrial pressure vs L) At birth, lungs expand > R atrial pressure drops > L atrial pressure closes flap o Morphology (classified according to location) Secundum ASD (90%) > deficient septum secundum formation Primum anomalies (5%) > adjacent to AV valves Sinus venosus defects (5%) > near entrance of SVC o Clinical Features ASDs result in L > R shunt Pulmonary blood flow up to 2-8x normal Murmur often present Generally well tolerated, and nonsymptomatic before age 30 Irreversible pulmonary hypertension is unusual Mortality is low, and long-term survival is comparable to normal population o Patent Foramen Ovale Closes permanently in 80% of people by age 2 Can open in remaining 20% from sustained pulmonary hypertension Ventricular Septal Defect (VSD) o Most common types of CHD o Morphology (classified according to size and location) Membranous VSD (90%) > membranous septum, size of aortic valve orifice Infundibular VSD (10%) > below the pulmonary valve or in the muscular septum o Clinical Features Most pediatric VSDs associated with other anomalies (ex. Tetralogy of Fallot) If diagnosed in adult, they're usually isolated anomalies Functional consequence depends on size of hole and R side malformation 50% of small muscular VSDs close spontaneously Delayed treatment in hopes of spontaneous closure Large VSDs result in early R ventricular hypertrophy + pulmonary hypertension Lead to irreversible pulmonary vascular disease if not treated early Patent Ductus Arteriosus (PDA) o Joins pulmonary artery and aorta, distal to origin of L subclavian artery o Bypasses lungs during fetal life o Constricts and closes 1-2 days after birth due to increased arterial oxygenation, decreased pulmonary vascular resistance, and declining prostaglandin E o Complete obliteration within first few months > ligamentum arteriosum o Closure delayed in infants with hypoxemia o Produce characteristic "machinery-like" murmur o Should be closed as early as possible (easy surgery) unless required preserving for infants with other cardiac malformations (via administering prostaglandin E) Right-to-Left Shunts Produce hypoxemia and cyanosis Prolonged distal cyanosis can produce clubbing of finger tips "hypertrophic osteoarthropathy" Tetralogy of Fallot o VSD, Pulmonary stenosis, Overriding aorta, R ventricular hypertrophy o Morphology Heart enlarged and "boot-shaped" b/c of R ventricular hypertrophy Large VSD with aortic valve at superior border o Clinical Features Clinical consequences depend on severity of pulmonary stenosis Severe stenosis > more hypoplastic (smaller/thin walled) pulmonary arteries + larger overriding aorta Most infants are cyanotic at birth Obstruction becomes progressively worse with growth Surgery may be complicated Transposition of the Great Arteries o Aorta arises from R ventricle, Pulmonary artery from L ventricle o Atrial-Ventricular connections are normal (R to R, L to L) o Results in separation of systemic and pulmonary circulations > incompatible with postnatal life unless a shunt exists o Without surgery most patients die within months Tricuspid Atresia o Occlusion of the tricuspid valve orifice o Mitral valve is larger than normal + R ventricular hypoplasia o Requires extensive shunting to be compatible with life (ASD + VSD) o Cyanosis present from birth and associated with high early mortality Obstructive Lesions Coarcation of the Aorta o Infantile form: proximal to a PDA o Adult form: distal to arch vessels o 50% of cases accompanied by biscupid aortic valve and other CHD o Clinical Features Coarcation of aorta with PDA = cyanosis in lower half of body Req. surgical occlusion of PDA Coarcation of aorta w/o PDA = asymptomatic in childhood Collateral circulation = noticed by radiographic notching on ribs Hypertension in upper extremities Hypotension in lower extremities Pulmonary Stenosis and Atresia o Obstruction at pulmonary valve > R ventricular hypertrophy o Pulmonary trunk NOT dilated (may even be hypoplastic) Aortic Stenosis and Atresia o Valvular, subvalvular, or supravalvular Valvular = cusps hypoplastic, dysplastic (thickened), or abnormal in number o Obstruction of outflow tract > L ventricle hypoplasia (hypoplastic L heart syndrome) PDA has to be open to allow blood flow to aorta and coronary arteries Ischemic Heart Disease (aka Coronary Artery Disease - CAD) o Results from imbalance between myocardial supply (perfusion) and cardiac demand for O2 rich blood (ATP synth) Also limits availability of nutrients and removal of waste products o 90% results from reduced blood flow due to obstructive atherosclerotic lesions in coronary vessels A lesion obstruction >75% of vessel defines significant CAD (symptomatic threshold) Usually slow progression of lesions until sudden onset of symptoms Slow progression allows collateral vessels to develop over time Common occlusions occur in the LAD, LCX, and RCA o Can also be caused by coronary emboli, myocardial vessel inflammation, or vascular spasm Initiated by unpredictable and abrupt rupture/erosion/ulceration of atherosclerotic plaque Plaque changes precipitate formation of superimposed thrombus Thrombus partially or completely occludes the artery o o Epidemiology Leading cause of death in US Rate has fallen 50% since 60's due to prevention and diagnostic/therapeutic advances Effects of obesity boom to be determined Consequence of Myocardial Ischemia Angina Pectoris Paroxysmal and recurrent attacks of substernal/precordial chest discomfort Caused by transient (15sec to 15min) myocardial ischemia that is insufficient to induce necrosis Interplay of decreased perfusion, increased demand, and coronary artery pathology Stable (aka Typical) Angina o Imbalance in coronary perfusion relative to myocardial demand o Produced by physical activity, excitement, or stress o Deep, poorly localized pressure, squeezing, or burning sensation o Usually relieved by rest (dec demand) or administering vasodilators (inc perfusion) Prinzmetal Variant Angina (uncommon) o Caused by coronary artery spasm o Angina attacks unrelated to physical activity, heart rate, or pressure o Respond well to vasodilators Unstable (aka Crescendo) Angina o Increasingly frequent, prolonged (>20min), or severe angina o Frank pain, triggered by progressively lower levels of physical activity o Usually caused by disruption of atherosclerotic plaque with superimposed partial thrombosis and possibly embolization and/or vasospasm o 1/2 of patients have evidence of myocardial necrosis.. acute MI may be imminent Myocardial Infarction Death of cardiac muscle due to prolonged severe ischemia Coronary Artery Occlusion (90% of cases) o Atheromatous plaque undergoes acute change (intraplaque hemorrhage, erosion, etc.) o Platelets adhere to subendothelial collagen and necrotic plaque content > microthrombi o Vasospasm is stimulated from platelet granule release o Tissue factor activate coagulation pathway > add bulk to thrombus o Thrombus expands to completely occlude vessel in minutes Other possible triggers (10% of cases) o Vasospasm w/ or w/o atherosclerosis (due to platelet aggregation or drug ingestion) o Emboli from L atrium w/ atrium fibrillation or paradoxical emboli from R side of heart o Ischemia without detectable/significant coronary atherosclerosis and thrombosis Disorders of small intramural coronary vessels Hematologic abnormalities (sickle cell) Amyloid deposition in vascular walls Lowered systemic blood pressure (shock) Inadequate myocardial protection during cardiac surgery Time of Onset of Key Events caused by Ischemia o Cessation of aerobic metabolism within seconds > drop in ATP o Myocardial contractility ceases <2 minutes of severe ischemia o 50% normal ATP by 10 minutes, 10% normal ATP by 40 minutes o Severe ischemia (<10% blood flow) lasting 20-40 minutes leads to necrosis (irreversible) Occurs first in subendocardium o Damage to microvasculature after 1 hour o Damage to 1/2 myocardium thickness within 2-3 hours o Damage throughout myocardium within 6 hours Extent of Tissue Damage dictated by: o Location, severity, rate of development of coronary obstruction o Size of vascular bed perfused by obstructed vessel o Duration of occlusion o Metabolic/oxygen needs of myocardium o Extent of collateral blood vessels o Presence, site, and severity of coronary arterial spasm o Heart rate, cardiac rhythm, and blood oxygenation Arteries and areas of Blood Supply o Left Anterior Descending coronary artery (40-50%) Apex of heart, anterior wall of L ventricle, anterior 2/3 of ventricular septum o Right coronary artery (right dominant circulation - 80% of people) (30-40%) R ventricular free wall, posterobasal wall of L ventricle, posterior 1/3 of ventricular septum o Left Circumflex coronary artery (assuming right dominant circulation) (15-20%) Lateral wall of L ventricle except at the apex Patterns of Infarction o Transmural Infarction Caused by occlusion of an epicardial vessel (w/o therapeutic intervention) Full thickness of ventricular wall Associated with chronic coronary atherosclerosis, acute plaque change, and superimposed thrombosis o Subendocardial (nontransmural) Infarction Subendocardial zone is least perfused > most vulnerable Typically involves inner 1/3 of ventricular wall Can be initial stages of transmural infarction or as a result from prolonged severe hypotension (circumferential in the latter case) o Multifocal Microinfarction When pathology involves only smaller intramural vessel May occur from microembolism, vasculitis, or vascular spasm Spasms exacerbated by catecholamines or certain drugs Vasospasm can lead to sudden cardiac death or ischemic dilated cardiomyopathy (aka takotsubo cardiomypothy - broken heart syndrome) Morphology o Appearance of infarct depends on duration of survival following MI o Highlight areas of necrosis by submerging tissue slices in triphenyltetrazolium chloride Brick-red on noninfarcted myocardium Infarct areas appear unstained and pale (no lactate dehydrogenase activity) o 6-12 hours: wavy fibers at periphery of infarct o 12-24 hours: reddish-blue discoloration (trapped blood) o 1-3 days: nectoric muscle elicits acute inflammation o 3-7 days: macrophages remove nectoric myocytes o 10-14 days: rimmed by hyperemic zone of highly vascularized granulation tissue o 2-8 weeks: fibrous scar o Once completely healed age is impossible to determine (8 weeks looks like 10 years) Infarct Modification by Reperfusion o Restoration of blood flow in order to salvage cardiac muscle at risk > limit infarct size o Prompt reperfusion is the preeminent objective for treatment of MI patients o Accomplished by: thrombolysis, angioplasty, stent placement, CABG o Salvages reversibly injured cells + alters morphology of necrotic cells (contraction bands) o Can trigger lethal complications: arrhythmias, endothelial swelling (occlude capillaries) o Biochemical abnormalities persist for days-weeks > "stunned" myocardiumm Clinical Features o Clinical symptoms Prolonged (>30min) chest pain "crushing, stabbing, or squeezing" Rapid, weak pulse Profuse sweating (diaphoresis) Nausea or vomiting Dyspnea due to pulmonary congestion/edema o Presence of myocardial proteins in plasma Cardiac-specific Troponins T and I (cTnT and CtnI) MB fraction of creating kinase (CK-MB) CKMB, cTnT, and CtnI elevates first 3-12 hours CKMB and cTnI peak at 24 hours CKMB returns to normal in 48-72 hours cTnI returns to normal in 5-10 days cTnT returns to normal in 5-14 days o Characteristic electrocardiogram changes ST tombstones Consequences and Complications of Myocardial Infarction o Poor prognosis with advanced age, female gender, diabetes mellitus, and previous MI o Contractile dysfunction o Arrythmias 1/2 of deaths from acute MI occur within 1 hour due to fatal arrhythmia o Myocardial rupture Usually when there is transmural necrosis in ventricle Rupture of ventricular free wall with hemopericardium and cardiac tamponade Rupture of ventricular septum > acute VSD > L to R shunting Papillary muscle rupture > severe mitral regurgitation o Ventricular aneurysm o Pericarditis o Infarct expansion o Mural thrombus o Papillary muscle dysfunction o Progressive late heart failure Chronic Ischemic Heart Disease (aka Ischemic Cardiomyopathy) Progressive congestive heart failure due to accumulated ischemic myocardial damage Usually involves prior MI or coronary arterial interventions/surgery Usually appears after MI due to decompensation of hypertrophied noninfarcted myocardium Morphology: cardiomegaly, L ventricular hypertrophy/dilation, stenotic coronary atherosclerosis, scars from old infarcts