* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Organic Functional Groups Organic Functional Groups
Physical organic chemistry wikipedia , lookup
Homoaromaticity wikipedia , lookup
Metal carbonyl wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Organosulfur compounds wikipedia , lookup
Hydroformylation wikipedia , lookup
Asymmetric induction wikipedia , lookup
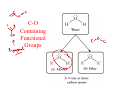
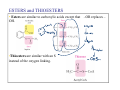
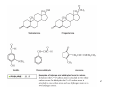
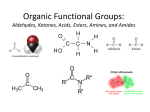
Organic Functional Groups Chapter 7 Alcohols, Ethers and More 1 What do you do when you are in Pain? • What do you do when you are in a lot of pain? 2 Functional Groups • A functional group is an atom, groups of atoms, or bond that gives a molecule a particular set of chemical properties. • Each family of organic compounds is defined by the functional group that its members contain. 3 C-O Containing Functional Groups 4 Alcohols All alcohols contain a hydroxyl (-OH) functional group that is attached to an alkane-type carbon atom. 5 Alcohols • Classification of alcohols is based on the degree of substitution of the carbon they are attached to. • Reactivity of alcohols is based on access. 6 Naming Alcohols • Drop the “e” ending on the name of the corresponding hydrocarbon replacing with “ol.” • The position of the -OH group must be specified with a number similar to the double bond. CH3 OH H3C H3C CH3 Is 2-methylcyclohexanol a primary, secondary or tertiary alcohol? 7 Properties of Alcohols • Type of Intermolecular Forces present? • Solubility in water? • Boiling Point? a. CH3CH2OH b. HO-CH2CH2OH c. . CH3CH3 d. CH3CH2CH2OH 8 Polarity vs Chain Length Octanol is insoluble in water due to the long nonpolar chain. Methanol, ethanol and the propanols are soluble in water due to the polar -OH. Properties of Alcohols 10 Ethers • An oxygen squeezed between carbons. • The simplest will be having two methyl groups attached. • To name ethers: • For simple ones name the groups in alphabetical order followed by the word ether. • When other functional groups are present the OR group has the ending –oxy as a substituent. -OCH3 is methoxy. • Solubility: • Hydrophobic or hydrophilic? • Polar or nonpolar? 11 Carbonyl Containing Functional Groups 12 THE CARBONYL GROUP • The carbonyl group is an oxygen double bonded to a carbon. • The carbon is connected to two groups or atoms. • One of these may be an R group and the other may be either an R group, a hydrogen or a heteroatom. • The carbonyl group is not a functional group, but is a component of functional groups shown on the following slide. Electronic Geometry? CARBONYL-CONTAINING GROUPS All are polar. Identify what is at each side of the carbonyl. Which ones have Hydrogen bond? Solubility in water? Aldehydes and Ketones 15 Naming Aldehydes and Ketones • When naming aldehydes and ketones the carbonyl (C=O) must be part of the parent chain and receive the lowest number. • Since the carbonyl of an aldehyde is always in position number 1, its position is not specified. • For ketones, however, the position of the carbonyl must be given, unless the molecule is small enough that there is no question as to carbonyl placement. • Parent chains are named by dropping the final “e” from the name of the corresponding hydrocarbon and adding “al” for aldehydes or “one” for ketones. 16 Aldehydes and Ketones 17 What is the name? O CH3 H3C CH3 a. b. c. d. e. 5-pentanone 2-pentanone 2-heptanal 3-ethylpentanone 3-ethyl-2-pentanone 18 • Aldehydes and ketones have much lower boiling points than alcohols with a similar molecular weight. • The differences in boiling points is due to the fact that alcohols can form hydrogen bonds while aldehydes and ketones cannot. • The C=O is slightly polar, which allows an aldehyde or ketone to interact with one another through dipole-dipole forces. 19 The polarity of the carbonyl group and its ability to form hydrogen bonds with water molecules allows small aldehydes and ketones to be highly water soluble (“like dissolves like”). 20 21 CARBOXYLIC ACIDS 22 Carboxylic acids contain a carboxyl functional group, which is the combination of a hydroxyl (-OH) group and a carbonyl (C=O) group. 23 Naming • All the rules learned with the ending -oic acid. • Carboxylic acids have priority number one in naming. H3C O H3C OH CH3 24 IONIZATION OF CARBOXYLIC ACIDS • The carboxylic acid group may lose a proton (H+) in solution to form the carboxylate ion. The name of the ion ends in –ate ion, as indicated. •Carboxylate ions are also much more soluble in water than their carboxylic acid counterparts. •What type of intermolecular force will be present? FATTY ACIDS AND SOAP • Remember that Fatty acids are long hydrocarbon chains containing the carboxylic acid group. • Soaps are the carboxylate form of fatty acids. • The fatty acid portion of soap dissolves into greasy materials, while the carboxylate portion attracts water, explaining the cleaning action of soap. HYDROGEN BONDING • Carboxylic acids easily form hydrogen bonds with water or other similar compounds. •Small chains will be soluble in water. • Their hydrogen bonds also increase their boiling points. What has the lowest boiling point? a. CH3OCH3 b. CH3CH2OH c. CH3CH2COOH d. CH3COCH3 Functional Groups 28 CARBOXYLIC ACID DERIVATIVES • Carboxylic acid derivatives resemble carboxylic acids in that they have a heteroatom connected to the carbonyl group. ESTERS and THIOESTERS • Esters are similar to carboxylic acids except that –OR replaces – OH. •Thioesters are similar with an S instead of the oxygen linking. Identify all functional groups present 31 AMIDES • Amides consist of a nitrogen bonded to a carbonyl carbon. • The nitrogen could have up to two hydrogens or R groups attached. •Naming: • Count the carbons and use the ending –anamide. •If the nitrogen is substituted named them as substituent starting with the letter (N-) to indicate the position. This image cannot currently be display ed. N-methylethanamide SOME COMMON AMIDES • Proteins and oligopeptides contain the amide group. • Penicillin is also an amide. OTHER FUNCTIONAL GROUPS Amines and Phosphates 34 AMINES • Amines are derived from ammonia (NH3) when hydrogens are substituted by carbon compounds (R). • What is the electronic geometry of ammonia? •Molecular geometry? • Amines are classified by the level of substitution: AMINES ARE NOT AMIDES! • Amines do not have a carbonyl group directly attached to the N. • This makes their properties entirely different. • One property of amines is that they form ions; amides cannot do this. NAMING AMINES • Each R group is treated like a usual substituent, and each ends with a –yl. • Following the substituent names in alphabetical order is the term “amine.” PHARMACOLOGICALLY ACTIVE NAMES • Many amines have active biological properties. • Amines derived from plants are called alkaloids. • Some alkaloids are shown below. HYDROGEN BONDING AND AMINES • Amines easily form hydrogen bonds. • As before, these determine physical properties such as boiling point and solubility. AMINES COULD FORM IONS • The nonbonding electrons on an amine nitrogen may attract a proton to give the nitrogen a fourth bond. • The resulting positively-charged polyatomic ion is analogous to the ammonium ion. • These are soluble and nature. common in AMINO ACIDS • Amino acids contain both the amino group and the carboxylic acid group. • In neutral solution, both are charged. • Amino acids are found in all proteins. PHOSPHATES • Phosphate esters are compounds derived from phosphoric acid. • All contain a P=O double bond and three P-O single bonds. The oxygens in a phosphate ester are joined to up to three R groups, replacing H’s. • The phosphate is usually present as a polyatomic ion with up to three negative charges. CLASSES OF PHOSPHATE ESTERS • The types of phosphate esters depend on the number of R groups present. • In the di- or tri-phosphate esters, the phosphate group is also attached to an oxygen of the next phosphate. This structure is termed a phosphoanhydride. ATP • ATP (adenosine triphosphate) is an example of a triphosphate ester. It is the energy currency of the cell, used as an intermediate in energy transfers. • Note the presence of two phosphoanhydrides in ATP. CHAPTER 7: Organic Functional Groups PRACTICE PROBLEM Epinephrine (adrenaline) is a hormone secreted when the body is under stress. Illicit drugs that are structurally similar to epinephrine are highly addictive stimulants. For example, the illegal and addictive drug methamphetamine is similar in structure to epinephrine. However, pseudoephedrine, which is structurally similar to epinephrine, is a decongestant and sold over the counter in pharmacies. a. Circle and label all the amine functional groups in these molecules. b. In what way is methamphetamine structurally similar to pseudoephedrine? How is it different? c. In the body, the amine in epinephrine is in its ionic form. Write the structure of the ionic form of epinephrine. 46 47 48 Compared to hydrocarbons with a similar molecular weight, alcohols have relatively high boiling points. 49 50