* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download the Alkali Metals
Survey
Document related concepts
Transcript
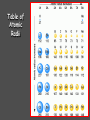
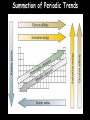
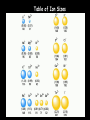
Chemistry Chapter 5&6 The Periodic Law Notes 5 Mendeleev’s Periodic Table Dmitri Mendeleev Modern Russian Table Chinese Periodic Table Stowe Periodic Table A Spiral Periodic Table Triangular Periodic Table “Mayan” Periodic Table Orbital filling table Periodic Table with Group Names The Properties of a Group: the Alkali Metals Easily lose valence electron (Reducing agents) React violently with water Large hydration energy React with halogens to form salts Sublevel Blocks of the Periodic Table Figure 5-5 p. 129 s-block • Group 1 • Alkali Metals – – – – ns1 is highest level Silvery appearance Soft—cut with knife Highly reactive—never found free in nature – Low melting points <100oC • Group 2 • Alkaline-earth metals – ns2 is highest level – Harder & denser, w/ higher melting points than Group 1 – Highly reactive—never found free in nature Special exceptions to s-block • Hydrogen – Has ns1 – Totally different properties from alkali metals • Helium – Has ns2 – Highest level is completely full – Stable like noble gases d-block • d sublevel for preceding energy level is filling • d sublevel filling has some deviations—Group 11: Cu, Ag, Au – Outer s & d sublevels still have same # e- • Transition elements: d-block metals w/ typical metallic properties – – – – Less reactive than Group 1 & 2 Exist free in nature Good conductors of electricity High luster (shiny) • • • • p-block All elements of Groups 13-18 except Helium Properties vary greatly Nonmetals (right hand end) All six metalloids – Brittle solids – Some properties of metals and nonmetals • Eight metals (left hand side and bottom of the block) – Harder and denser then s-block alkaline-earth metals – Softer and less dense than d-block metals – Stable in the presence of air • Group 17 Halogens – Most reative of the nonmetal – 7 electrons in outer shell f-block • Lanthanides & Actinides • 14 elements—seven 4f orbitals are filling • Lanthanides – Similar reactivity to Group 2 – Shiny metals • Actinides – Only 1st four found in nature – All are radioactive Determination of Atomic Radius: Half of the distance between nucli in covalently bonded diatomic molecule "covalent atomic radii" Periodic Trends in Atomic Radius Radius decreases across a period Increased effective nuclear charge due to decreased shielding Radius increases down a group Addition of principal quantum levels Table of Atomic Radii Ionization Energy - the energy required to remove an electron from an atom Increases for successive electrons taken from the same atom Tends to increase across a period Electrons in the same quantum level do not shield as effectively as electrons in inner levels Irregularities at half filled and filled sublevels due to extra repulsion of electrons paired in orbitals, making them easier to remove Tends to decrease down a group Outer electrons are farther from the nucleus Ionization of Magnesium Mg + 738 kJ Mg+ + eMg+ + 1451 kJ Mg2+ + eMg2+ + 7733 kJ Mg3+ + e- Table of 1st Ionization Energies Another Way to Look at Ionization Energy Electron Affinity - the energy change associated with the addition of an electron Affinity tends to increase across a period Affinity tends to decrease as you go down in a Group or family Electrons farther from the nucleus experience less nuclear attraction Some irregularities due to repulsive forces in the relatively small p orbitals Table of Electron Affinities Ionic Radii Cations Anions Positively charged ions Smaller than the corresponding atom Negatively charged ions Larger than the corresponding atom Summation of Periodic Trends Table of Ion Sizes Electronegativity A measure of the ability of an atom in a chemical compound to attract electrons Electronegativities tend to increase across a period Electronegativities tend to decrease down a group or remain the same Periodic Table of Electronegativities