* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Spin Quantum Number - stpats-sch3u-sem1-2013
Quantum field theory wikipedia , lookup
Spin (physics) wikipedia , lookup
Particle in a box wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Double-slit experiment wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
Many-worlds interpretation wikipedia , lookup
Quantum dot wikipedia , lookup
Quantum fiction wikipedia , lookup
Wave–particle duality wikipedia , lookup
Quantum entanglement wikipedia , lookup
Tight binding wikipedia , lookup
Quantum computing wikipedia , lookup
Chemical bond wikipedia , lookup
Molecular orbital wikipedia , lookup
Ferromagnetism wikipedia , lookup
Orchestrated objective reduction wikipedia , lookup
Bell's theorem wikipedia , lookup
Interpretations of quantum mechanics wikipedia , lookup
Quantum teleportation wikipedia , lookup
Canonical quantization wikipedia , lookup
Quantum machine learning wikipedia , lookup
Quantum key distribution wikipedia , lookup
Symmetry in quantum mechanics wikipedia , lookup
Quantum group wikipedia , lookup
History of quantum field theory wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
Hidden variable theory wikipedia , lookup
EPR paradox wikipedia , lookup
Quantum state wikipedia , lookup
Atomic theory wikipedia , lookup
Hydrogen atom wikipedia , lookup
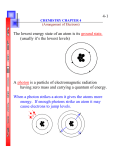
Atomic Orbitals and Quantum Numbers Electrons are found in atomic orbitals, or atomic space, surrounding the nucleus of an atom. For a neutral atom, the number of electrons in the element is equal to the atomic number. For example, nitrogen’s atomic number is 7 and so, an atom of nitrogen has 7 electrons. However, these 7 electrons do not coexist in the same orbital. Hund’s rule states that there can only be a maximum of two electrons in one orbital and that each of these electrons have opposite spins to each other. Furthermore, If there are more electrons after the 1s, and 2s orbitals have been filled, each p orbital will be filled with one electron first before two electrons try to reside in the same p orbital. How do these electrons and their orbitals divide themselves around the nucleus of an atom? The electrons are given quantum numbers. These quantum numbers are like an address for the electron. Each quantum number has a particular characteristic. There are four quantum numbers: the principal quantum number, second quantum number, magnetic quantum number and the spin quantum number. Principal Quantum Number The collection of orbitals in a principal quantum number is called an electron shell. Once the first electron shell has been filled to its maximum number of allowable electrons, the next electron shell starts to fill up. The electron shell is identified as n and has values of 1, 2, 3, and so on. These n values also correspond to the number of types of subshells and their shape. Electrons found in the electron shells furthest from the nucleus are not strongly bonded to the nucleus and have higher energies. Second Quantum Number The second quantum number refers to the number of subshells and their respective shapes. The subshell designations are s, p, d, f and theoretically each of these subshells can contain a maximum number of electrons. Magnetic Quantum Number The magnetic quantum number refers to the orientation of the orbital of the subshell. Spin Quantum Number The spin quantum number refers to the spin of the electron in the orbital. Like charges repel. In order for 2 electrons to occupy an orbital, they each have to spin in opposite directions about their own axis.