* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download PPT File
Oxidation state wikipedia , lookup
Hydroformylation wikipedia , lookup
Cluster chemistry wikipedia , lookup
Metal carbonyl wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Jahn–Teller effect wikipedia , lookup
Metalloprotein wikipedia , lookup
Spin crossover wikipedia , lookup
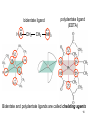
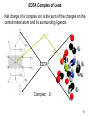
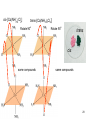
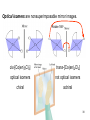
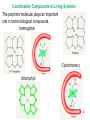
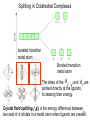
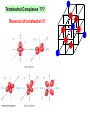
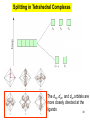
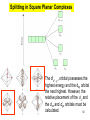
University Chemistry Chapter 15: The Chemistry of Transition Metals Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. What are the transition metals ? Element with typical electron configurations ns2 (n-1)dx incompletely filled d orbitals Properties “metallic” due to loosely bound ns electrons. Various colors Various reduction/oxidation potentials Possess catalytic activities 3 4 17.1 The d-block Metals: Energies, Charge States and Ioinc Radii Charge states (oxidation states) Early transition metals Late transition metals High oxidation states do not mean the ionic charges Early d elements: of the metals. Formation of oxyions Just oxidation by polar-covalent numbers! bonding with O, Cl, F Late d elements: resistant to further oxidation beyond M2+ or M3+ * Metal ions (mostly coordination complex ions) ** Ionization Energies for the 1st Row Transition Metals 6 17.1 The d-block Metals: Energies, Charge States and Ioinc Radii Ionic radii Similar to neutral ones. Not a so smooth change as for the p elements. 17.2 chemistry of the early transition metals: oxyions Oxyions : metals combined with oxygen to form a polyatomic molecular ion. examples Cr2O72- MnO4- Oxidizing agents permanganate dichromate CrO42- + 2H+ Cr2O72- + H2O H2SO4 CrO3 Sc2O3 TiO2 H2O H2CrO4 V2O5 Oxidation states: higher oxidation state– more covalent bond character lower oxidation state – more ionic bond character Mn(OH)2, Mn(OH)3, H2MnO3, H2MnO4, HMnO4 basic acidic 17.2 chemistry of the early transition metals: oxyions Spectroscopy and structure of oxyanions Comparison of isoelectronic species VO4 3- CrO4 2- MnO4 Follows octet rule. Tetrahedral structure according to VSEPR. - Absorption spectra * 17.2 chemistry of the early transition metals: oxyions VO43- CrO42- MnO4- Molecular orbitals 4p and 4s AO are spatially more extended than 3d, to interact with oxygen MO’s. 3d AO’s remain as nonbonding. follow octet rule, Tetrahedral structure 17.2 chemistry of the early transition metals: oxyions VO43- CrO42- MnO4- follow octet rule, Tetrahedral structure Molecular orbitals Charge-transfer transitions : electron jumps from O to M like orbitals. This can absorb light very strongly. 17.3 chemistry of the late transition metals: coordination complexes Stoichiometry, Isomerism, and Geometry of Complexes Chemical formula(19thC.) Color Chemical formula (Werner) Isomers CoCl3.6NH3 orange-yellow [Co(NH3)6]3+Cl-3 1 CoCl3.5NH3 purple [Co(NH3)5Cl]2+Cl-2 1 CoCl3.4NH3 green [Co(NH3)4Cl2]+Cl- 2 CoCl3.3NH3 green [Co(NH3)3Cl3] 1 Octahedral structure cis trans These two are geometrical isomers 17.3 chemistry of the late transition metals: coordination complexes Oxidation numbers of 2 and 3 (1 is possible for Cu). Formation of common oxides: Fe3O4, Fe2O3, CoO, Co3O4, NiO, Cu2O, CuO, ZnO Oxides are easily soluble in acid to form colored solution. d10(Cu, Zn) are colorless. Color of aqueous solution of ions Fe2+(aq) Fe3+(aq) Co2+(aq) Co3+(aq) CuSO4 : greenish white v.s. Ni2+(aq) Cu2+(aq) CuSO4.4H2O : blue These colors are due to the formation of coordination complexes. Coordination complex : Cu(H2O)42+ n+ M n+ + mL Ligand coordination Making coordination complex charge of a complex = sum of charges of metals and ligands charge of a complex + charges of counter ions = 0 coordination number = numbers of donor atoms M Lm coordination complex m : coordination number Coordination Compounds A coordination compound typically consists of a complex ion and a counter ion. Primary valence corresponds to the oxidation number and secondary valence to the coordination number of the element. A complex ion contains a central metal cation bonded to one or more molecules or ions. The molecules or ions that surround the metal in a complex ion are called ligands. H H H H H •• Cl •• - C •• O •• •• •• N •• A ligand has at least one unshared pair of valence electrons O 14 The atom in a ligand that is bound directly to the metal atom is the donor atom. •• N O H H H H H The number of donor atoms surrounding the central metal atom in a complex ion is the coordination number. Ligands with: one donor atom two donor atoms three or more donor atoms monodentate bidentate H2O, NH3, Clethylenediamine polydentate EDTA 15 bidentate ligand •• H2N CH2 CH2 •• NH2 polydentate ligand (EDTA) Bidentate and polydentate ligands are called chelating agents 16 17 EDTA Complex of Lead Net charge of a complex ion is the sum of the charges on the central metal atom and its surrounding ligands. Pb2+ EDTA4- Complex: 218 19 17.3 chemistry of the late transition metals: coordination complexes Nomenclature 1. Cation Anion b 2. In the complex: names of ligands come first and then name of metal. Among ligands: alphabetical order. 3. Names of ligands: anion – change the last letter to o. neutral – same as the original ones. 4. Counting number of ligands: di, tri, tetra, penta, hexa, hepta….. if the ligand contains these names in it, use: bis, tris, tetrakis, pentakis…… 5. If the compex is an anion: at the end of the name put ate. 6. Oxidation number of metal: in parenthesis with Roman letter - (IV). Examples [Co(NH3)5Cl]Cl2 Pentaamminechlorocobalt(III) chloride K4[Fe(CN)6] Potassium hexacyanoferrate(II) Naming Coordination Compounds • The cation is named before the anion. • Within a complex ion, the ligands are named first in alphabetical order and the metal atom is named last. • The names of anionic ligands end with the letter o. Neutral ligands are usually called by the name of the molecule. The exceptions are H2O (aquo), CO (carbonyl), and NH3 (ammine). • When several ligands of a particular kind are present, the Greek prefixes di-, tri-, tetra-, penta-, and hexa- are used to indicate the number. If the ligand contains a Greek prefix, use the prefixes bis, tris, and tetrakis to indicate the number. • The oxidation number of the metal is written in Roman numerals following the name of the metal. • If the complex is an anion, its name ends in –ate. 21 22 23 24 25 Structure of Coordination Compounds Coordination number 2 Structure Linear 4 Tetrahedral or Square planar 6 Octahedral 26 17.3 chemistry of the late transition metals: coordination complexes Structure of coordination complexes [Ag(NH3)2]+ coordination number 2 structure Linear Atomic orbital of metal d10 4 Tetrahedral d10 [Pt(NH3)4]2+ 4 Square Planar d8 [Co(NH3)6]3+ 6 Octahedral d6 [Zn(NH3)4]2+ *(b) and (c) are geometrical isomers. Stereoisomers are compounds that are made up of the same types and numbers of atoms bonded together in the same sequence but with different spatial arrangements. Geometric isomers are stereoisomers that cannot be interconverted without breaking a chemical bond. cis-[Pt(NH3)2Cl2] trans-[Pt(NH3)2Cl2] 28 cis-[Co(NH3)4Cl2] trans-[Co(NH3)4Cl2] Rotate 90o Rotate 90o trans cis same compounds same compounds 29 Optical isomers are nonsuperimposable mirror images. Rotate 180o cis-[Co(en)2Cl2] trans-[Co(en)2Cl2] optical isomers not optical isomers chiral achiral 30 17.3 chemistry of the late transition metals: coordination complexes Multidentate ligands (or chelating ligands) bidentate ligands tetradentate hexadentate These are non-superimposable mirror images to each other. So they are optical isomers. Coordination Compounds in Living Systems The porphine molecule plays an important role in some biological compounds. hemoglobin Cytochrome c chlorophyll 32 17.3 chemistry of the late transition metals: coordination complexes Magnetism: paramagetic v.s. diamagnetic (paramagnetic if there are unpaired electrons) Zn2+ d10 always diamagnetic – no unpaired electrons [Co(NH3)6]3+ d6 diamagnetic – no unpaired electrons low spin complex [CoF6]3- d6 paramagnetic – 4 unpaired electrons high spin complex Lability: ability to undergo a reaction (should meet both the kinetic and thermodynamic requirements). Some ligands bind to metals more tightly than others, replacing weakly bound ligands. Replacement of a strong ligand by a weak one is labile. CO, CN-, H2NCH2CH2NH2 > H2O [Ni(H2O)6]2+ + 6NH3 [Ni(NH3)6]2+ + 6H2O [Ni(en)3]2+ + 6H2O Kf = 4 x 108 [Ni(H2O)6]2+ + 3en Kf = 2 x 1018 Kf is called the formation constant of a complex (see Table 17.3). 17.4 The spectrochemical series and bonding in complex Color changes of complexes NiCl2 HCl(12M) NiCl64- H2O Ni(H2O)6 2+ NH3 Ni(NH3)62+ en Ni(en)32+ more stable Spectrochemical Series: Arrangement of ligands in order of increasing stability of complex. Magnetic properties also follow this series. strong field ligands weak field ligands Example: Fe(H2O)62+ paramagetic a high-spin complex Example: Fe(CN)64diamagnetic a low-spin complex Strong-field ligands form a high-spin complex that are paramagnetic, whereas weak-field ligands form a paramagnetic low-spin complex. 17.4 The spectrochemical series and bonding in complex Are there ways to explain and eventually predict colors, spectrochemical series and magnetism? Crystal field theory : ionic description of the metal-ligand bonds Only considering the electrostatic interaction between ligand and metal atom: charge-charge, charge-dipole. Then, consider changes in the energy levels of metal d orbitals according to the interaction during coordination. Let’s Begin with octahedral geometry Bonding in Coordination Compounds Crystal field theory explains the bonding in complex ions purely in terms of electrostatic forces. • the attraction between the positive metal ion and the negatively charged ligand or the partially negatively charged end of a polar ligand • electrostatic repulsion between the lone pairs on the ligands and the electrons in the d orbitals of the metals All d orbitals equal in energy in the absence of ligands! 37 Splitting in Octahedral Complexes Isolated transition metal atom Bonded transition metal atom The lobes of the d x 2 y 2 and d 2are z pointed directly at the ligands, increasing their energy. Crystal field splitting ( D ) is the energy difference between 38 two sets of d orbitals in a metal atom when ligands are present Crystal field theory for the octahedral structure Do : crystal field splitting energy Overall stabilization through splitting: crystal field stabilization energy (CFSE) Color of Coordination Compounds Absorbs all wavelengths: Black Transmits all wavelengths: Colorless (white) If one color is absorbed, the complementary color is seen. A solution of CuSO4 absorbs orange wavelengths so the solution appears blue. Energy of absorbed photon = D 40 Different values of D, result in different colors exhibited by complex ions. Aquo complexes of first row transition metal ions. Ti3+ Cr3+ Mn2+ Fe3+ Complexes will be colorless if no light is absorbed or if the absorbed wavelength is not in the visible region. Co2+ Ni2+ Cu2+ 41 Spectroscopic Determination of D l=498 nm 42 Spectrochemical Series A list of ligands arranged in increasing order of their abilities to split the d-orbital energy levels. I- < Br- < Cl- < OH- < F- < H2O < NH3 < en < CN- < CO increasing D Weak-field ligands Small D Strong-field ligands Large D 43 Magnetic Properties weak-field ligand strong-field ligand The arrangement of the electrons is determined by the stability gained by having maximum parallel spins versus the investment in energy required to promote electrons to higher d orbitals. Actual number of unpaired electrons can be determined by electron spin spectroscopy ( ESR). 44 Orbital diagrams for the highspin and low-spin octahedral complexes corresponding to the electron configurations d4, d5, d6, and d7. No such distinctions can be made for d1, d2, d3, d8, d9, and d10. 45 Summary of Octahedral Complexes magnetism d1 ~ d5 : always paramagnetic d7 ~ d9 : always paramagneticd6 : d10 : always diamagnetic depending on the ligands Tetrahedral Complexes ??? Reversal of octahedral !!! M Tetrahedral complexes Reversal of octahedral ! Splitting in Tetrahedral Complexes The dxy, dyz, and dxz orbitals are more closely directed at the ligands 49 Square planar??? Removal of axial ligands from octahedral Removal of axial ligands from octahedral Splitting in Square Planar Complexes The d x 2 y 2 orbital possesses the highest energy and the dxy orbital the next highest. However, the relative placement of the d z and the dxz and dyz orbitals must be calculated. 52 2 Weak point of crystal field theory 1. Coordination is not fully ionic. 2. Spectrochemical series is all empirical. 3. Does not consider nature of the ligands. Ligand field theory Ligand Field Theory Molecular orbital approach to the electronic structure of coordination compounds. Based on the idea that atomic orbitals that are close in energy will mix more effectively in molecular orbitals than those that are far apart. d x 2 y 2and d z 2 orbitals on the metal center will mix with the ligand lone-pair orbitals to form two bonding and two antibonding molecular orbitals. The remaining d orbitals—dxy, dyz and dxz—are oriented in between the ligands and will remain nonbonding orbitals 54 Arrangement of the highest occupied molecular orbitals (3dxy, 3dyz, 3dxz, and the two s*d orbitals) is identical to that predicted by crystal field theory Provides an understanding of the dependence of the crystal field splitting on the ligand type. 55 Now, we can explain Spectrochemical Series I- < Br- < Cl- < F-, OH- < H2O < NCS- < NH3 < en < CO, CNWeak field Ligands small D o Interaction between dxy of metal and py of halide: charge repulsion Increases energy level of t2g Strong field Ligands large D o pback-bonding of ligand can overlap pwith dxy orbital Lowers the energy level of t2g p back-bonding Makes smaller D o for I- and less smaller one for F- Makes larger Do for CO, CN- Ligand exchange (or substitution) reactions kinetic lability - tendency to react instantaneous *CN: 14C labeled labile complex—undergo rapid ligand exchange reactions. A thermodynamically stable species (that is, one that has a large formation constant) is not necessarily unreactive. several days inert complex—a complex ion that undergoes very slow exchange reactions A thermodynamically unstable species is not necessarily chemically reactive. 57 Applications of Coordination Compounds Metallurgy extract and purify metals, Therapeutic Chelating Agents EDTA is used in the treatment of lead poisoning. Certain platinum-containing compounds can effectively inhibit the growth of cancerous cells. 58 Chemical Analysis bis(dimethylglyoximato)nickel(II) dimethylglyoxime Characteristic colors are used in qualitative analysis to identify nickel and palladium. Detergents Tripolyphosphate ion is an effective chelating agent that forms stable, soluble complexes with Ca2+ ions. 59 Cisplatin – The Anticancer Drug Cisplatin works by chelating DNA (deoxyribonucleic acid), the molecule that contains the genetic code. Consequently, the double-stranded structure assumes a bent configuration at the binding site which is thought to inhibit 60 replication.