* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Histamine and Antihistaminic Agents
Survey
Document related concepts
Transcript
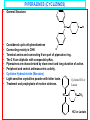
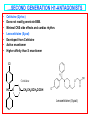
PHYSIOLOGICAL EFFECTS OF ENDOGENOUS HISTAMINE • Mediator of hypersensitivity • Regulation of gastric acid secretion • Neurotransmitter in the CNS • HISTAMINE H1-ANTAGONISTS • Antihistamines historically refer to antagonists at H1 receptors. • First generation or Classical H1-antagonists include: – – – – – • • • • Aminoalkyl ethers Piperazines Propylamines Phenothiazines Dibenzocycloheptenes/heptanes Second or Non-sedating generation Some structural similarities to first generation. More specific in action –lesser side effects. Limited in distribution profiles STRUCTURE ACTIVITY RELATIONSHIPS • • • • • • • • • • • • General Antihistamine Structure Ar = Aryl (phenyl, substituted phenyl, heteroaryl) Ar’ = Second aryl or arylmethyl; X = O, C or N; (CH2)n = carbon chain; NRR’ = Terminal amine group Subclassification of 1st generation antihistamines is based on nature of the connecting atom; diaryl substitution pattern; and the terminal amine function Diaryl substitution: is essential for significant H1 binding; present in 1st and 2nd generation antihistamines; must not be co-planar; may be tricyclic e.g. Phenothiazines, Dibenzocycloheptanes Amine Moiety: may be: simple dimethyl amino or part of heterocycle e.g. some Phenothiazines, Dibenzocycloheptenes, 2nd generation antihistamines, etc. (CH2)n , n = 2 or 3 Diaryl causes 1st and 2nd generation antihistamines to be more hydrophobic than histamine. Atihistamine Metabolism Oral administration results in 1st pass metabolism N-dealkylation; deamination Amino acid/glucuronide conjugation; aromatic hydroxylation to form phenols which can then be conjugated. Ar' R X Ar (CH2)n N R' AMINOALKYL ETHERS (ETHANOLAMINES) • • General structure: Diaryl tertiary aminoalkyl ether moiety is a shared pharmacophore for muscarinic receptors. Aminoalkyl ethers display anticholinergic activity. R'' Ar' O Ar • • • R CH2 CH2 N R' Diphenhydramine Hydrochloride (Benadryl): Displays anticholinergic and sedative properties Oral, IM and IV administration. CHOCH2CH2N(CH3)2 HCl Diphenhydramine Hydrochloride …AMINOALKYL ETHERS (ETHANOLAMINES) • Dimenhydrinate (Dramamine) • • • Used against motion sickness (30 min before trip) and Hyperemesis gravidarum (nausea of pregnancy). Oral, IM and IV administration. H CHOCH2CH2N(CH3)2 O Cl Dimenhydrinate N N N N CH3 CH3 O …AMINOALKYL ETHERS (ETHANOLAMINES) • • • • • • Doxylamine Succinate (Decapryn Succinate) Potent like diphendydramine Has sedating action Carbinoxamine Maleate (Clistin) Potent antihistamine Is a racemic mixture Cl CH3 CHCOOH CH2COOH CHCOOH CH2COOH OCH2CH2N(CH3)2 OCH2CH2N(CH3)2 N Doxylamine Succinate Carbinoxamine Maleate N …AMINOALKYL ETHERS (ETHANOLAMINES) • • • • Clemastine Fumarate (Tavist) Dextro clemastine has 2 chiral centers. Long duration of action Also has antimuscarinic activity …AMINOALKYL ETHERS (ETHANOLAMINES) • • Phenyltoloxamine Aromatic rings located differently from diphenhydramine yet produces potent antihistamine activity CHOCH2CH2N(CH3)2 HCl Diphenhydramine Hydrochloride PROPYLAMINES (MONOAMINOPROPYL DERIVATIVES) • General Structure: Ar' R CH • • • • C H2 C H2 N Pheniramines Ar R' Propylamines with saturated connecting carbon; consist of phenyl and 2pyridyl aryl group and terminal dimethylamino. All pheniramines are chiral. Antihistaminic activity almost exclusively on S-stereomers. Pheniramines differ in phenyl substitution at para position: H, Cl and Br. Halogenation yields 20-50-fold more potency. Poor oral availability – Firstpass metabolism. Metabolism by mono- & di-N-dealkylation and NOxidation & amino acid conjugation. …PROPYLAMINES (MONOAMINOPROPYL DERIVATIVES) • • • Chlorpheniramine Maleate (ChlorTrimeton). Dextro enantromorph is most active. Half-life of 12-15 hrs. Chlorpheniramine or Dexchlorpheniramine maleate HC CH2CH2N(CH3)2 N CHCOOH CHCOOH Cl …PROPYLAMINES (MONOAMINOPROPYL DERIVATIVES) • • • Triprolidine Hydrochloride Geometric isomerism important for activity. Trans isomers (pyrrolidinomethyl group is trans to the 2-pyridyl group) possess activity. (E)- isomers are superior to (Z)- isomers as H1 antagonists. Triprolidine HCl N C C H C N H2 HCl CH3 PIPERAZINES (CYCLIZINES) • General Structure: CH • • • • • • • • • N N R Considered cyclic ethylenediamines X Connecting moiety is CHN Terminal amine and connecting N are part of piperazine ring. The 2 N are aliphatic with comparable pKas. Piperazines are characterized by slow onset and long duration of action. Peripheral and central antimuscarinic activity. Cyclizine Hydrochloride (Marezine) Light-sensitive crystalline powder with bitter taste. Cyclizine HCl or Treatment and prophylaxis of motion sickness. Lactate HC N N CH3 HCl or Lactate …PIPERAZINES (CYCLIZINES) • Meclizine HCl (Bonine, Antivert). • Used mainly against nausea and vomiting associated with vertigo and motion sickness. Meclizine HCl CH3 HC N N HCl Cl CH2 PHENOTHIAZINES • General Structure: R S N CH2CH(CH2)N R'' • • • • • • R' Ethylenediamines with bridged aryl units 2 or 3 carbons between ring system and terminal N. Branched alkyl chain is required for the antihistaminic phenothiazines but not the antipsychotic phenothiazines. Chirality close to alkyl N has less effect on antihistaminic activity. Metabolism by mono- & di-N-dealkylation, aromatic oxidation and Noxidation. Promethazine Hydrochloride (Phenergan) Promethazine HCl S N CH2CHN(CH3)2 CH3 HCl DIBENZOCYCLOHEPTENES/HEPTANES • General Structure: X N CH3 • • • • Related to the phenothiazines except that the S and N atoms are replaced by 2 and 1 carbons, respectively. Cyproheptadine Hydrochloride (Periactin) Possesses both antihistamine and antiserotonin. Antipruritic agent. Sedation as principal side effect. SECOND GENERATION H1-ANTAGONISTS • • • • • Structurally dissimilar; similar physiological profiles; high potency H1-antagonists Prolonged antihistaminic effects due to slow receptor dissociation and active antihistaminic metabolites. Less affinity for muscarinic, adrenergic and serotonergic receptors. Therefore less sedating side effects. Serious arrhythmias when co-administered with drugs that inhibit their metabolism such as imidazole antifungals. …SECOND GENERATION H1-ANTAGONISTS • • • • Fexofenadine (Allegra) Oxidized metabolite of Terfenadine Less cardiotoxic than Terfenadine. Marketed as a racemate. • • • • Selective for H1 with no sedative other CNS effects. Experimental evidence shows it does not cross the BBB. 60-70% protein-bound, 5% is metabolized. Remainder is eliminated in bile and urine. OH C CH3 HO N COOH CH3 HCl Fexofenadine Terfenadine …SECOND GENERATION H1-ANTAGONISTS • • • • • • • • Loratadine Structurally related to dibenzocycloheptenes/heptanes. Selective peripheral H1-antagonist. No CNS or autonomic side-effects. Half-life of 8-15 hr. Desloratadine (Clarinex) Metabolite of Loratadine More potent inhibitor of histamine release Cl N Loratadine N COOCH2CH3 Desloratadine (Clarinex) …SECOND GENERATION H1-ANTAGONISTS • • • • • • • Cetirizine (Zyrtec) Does not readily penetrate BBB. Minimal CNS side effects and cardiac rhythm. Levocetirizine (Xyzal) Developed from Cetirizine Active enantiomer Higher affinity than S enantiomer Cl Cetirizine HC N N CH2CH2OCH2COOH Levocetirizine (Xyzal) TOPICAL HISTAMINE H1-RECEPTOR ANTAGONISTS • • • • • • Azelastine (Astelin) Nasal spray for allergic rhinitis N-dealkylated metabolite is active Eyedrops for allergic conjunctivitis Emedastine (Emadine) Topically for conjunctivitis Azelastine Emedastine TOPICAL HISTAMINE H1-RECEPTOR ANTAGONISTS • • • • • • • Levocabastine (Livostin) Conjunctivitis Olopatadine (Patanol) Structurally related to tricyclic antihistamines Long duration of action Rapid onset Nasal and eyedrops Levocabastine Olopatadine …HISTAMINE H2-RECEPTOR ANTAGONISTS • Histamine H2 antagonist are structurally related to histamine General Structure of H2 Receptor Antihistamines H N R NHCH 3 X HN N Y …HISTAMINE H2-RECEPTOR ANTAGONISTS • • • • • • • Cimetidine (Tagamet) Relatively hydrophilic. Affects Cytochrome P-450 metabolism of drugs. 60-70% oral bioavailability. Plasma half-life of ~2 hrs. Famotidine (Pepcid) Treatment of duodenal and benign gastric ulcers, gastroesophageal reflux disease, pathologic hypersecretory conditions and heartburn. H3C CH2SCH2CH2CNH2 CH2SHCH2CH2NHCNH2CH3 N NSO2NH2 NCN HN N Cimetidine (H2N)2C N S Famotidine …HISTAMINE H2-RECEPTOR ANTAGONISTS • • • • • • • Ranitidine (Zantac) HCl salt is highly water-soluble. Plasma half-life of 2-3 hr. Used to treat active duodenal ulcers associated with H. pylori infections. Nizatidine (Axid) Soluble in water, chloroform and alcohol. Half-life of 1-2 hr. CH2SCH2CH2NHCNHCH3 CHNO2 (H3C)2N Ranitidine O CH2SCH2CH2NHCNHCH3 CHNO2 S N Nizatidine (H3C)2N