* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download LUHS Handbook Approval
History of invasive and interventional cardiology wikipedia , lookup
Saturated fat and cardiovascular disease wikipedia , lookup
Cardiovascular disease wikipedia , lookup
Heart failure wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Remote ischemic conditioning wikipedia , lookup
Antihypertensive drug wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Cardiothoracic surgery wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Cardiac surgery wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Coronary artery disease wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Atrial fibrillation wikipedia , lookup
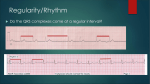
LOYOLA CARDIOLOGY Henri Matisse: © 2015 Succession H. Matisse / Artists Rights Society (ARS), New York INTRODUCTION Henri Matisse (1869-1954) The artwork featured on the front of this reference book was a work created by Henri Matisse titled “Icarus.” The myth arises from the story that Daedalus and his son Icarus, after revealing the secret of the Labarynth to the people of Greece were condemned to die in the Labarynth. They then devised a way to escape the maze by building wings of feathers and wax, but in his hubris and excitement, Icarus, failing to heed the warnings of his father, flew too close to the sun and the wings melted and fell to his death. Daedalus, having escaped to Siciliy, and his son’s body was found by Heracles who in turn burried him near a small rock promontory in the Aegean Sea. Matisse’s work is complex, showing Icarus in free fall, his death inevitable, but set against the bursts of sunlight and the surreal calm and peace depicted by the spot of red in place of his heart. Produced and Edited By Chris Latanich, MD (PGY 5) General Cardiology Fellow Contributors Shermeen Memon, MD (PGY 6) Mike Hushion, MD (PGY 6) Ambrose Panico, DO (PGY 5) Andrew Chen, MD (PGY 3) Chief Cardiology Fellow General Cardiology Fellow Chief Cardiology Fellow Internal Medicine Resident ADMISSIONS Admissions to the Cardiology Service: The following are guidelines regarding who is generally appropriate for admission to the cardiology inpatient service (as opposed to a general medicine service or ICU service), each patient must be evaluated respectively and if there is any question about the propriety of an admission, it should be discussed with the fellow on service or on call. 1) Complicated Heart Failure (e.g., severe edema, recurrent admissions or 30 day readmission, inotropic or LVAD support) 2) Intermediate Risk ACS 3) Severe Valvular Disease 4) Pericardial Disease 5) Post ACS Complications (chest pain, access problems etc...) 6) Non-Sustained Ventricular Tachycardia OR <1 ICD shocks 7) Cardiac Syncope 8) Hypertensive Urgency 9) Adult Congenital Heart Disease 10) Clinically Significant Arrhythmias Required Information For All Consults and Admissions: For any patient seen by the cardiology consult service and or admitted to the CCU / HTU / inpatient cardiology service, it is expected that the following records to have been obtained and in hand within 24 hours of the consultation / patient admission. Name and contact number of the patient’s primary cardiologist Angiogram (cath report) Transthoracic echocardiogram Stress test Lipid panel and A1c Baseline EKGs (or if a patient is admitted / transferred for VT / AT the arrhythmia in question) Open heart operative reports (bare minimum is the graft anatomy) Device interrogation report with indication for device implantation Ordering of Echocardiograms / Stress Tests: Before ordering a stress test it is imperative that you can provide some explanation of how the test will change your management of a given patient (don’t order a stress test on a patient who is 95 years old and on hospice for example). For echocardiograms, if one was done within the last six months, there needs to have been a clear change in clinical status to justify ordering it otherwise the study will not be reimbursed and the hospital or patient will be stuck with the cost. HYPERTENSION Hypertensive Urgency: Systolic BP > 180 mmHg or diastolic BP >110 mmHg without associated evidence of end organ damage. Treatment Goals: Avoid IV agents and high loading doses. Aim for a reduction in SBP to a target of 160/100 over a period of 6-12 hours then return to normal blood pressure in 24-48 hours time. Important to recognize that most of the patients live with very high blood pressures and so the risk is much higher if you drop their pressures too rapidly as they will likely have altered cerebral autoregulation. End Organ Damage Flash pulmonary edema Acute left ventricular failure Myocardial ischemia / infarction Stroke Subarachnoid hemorrhage Intracerebral hemorrhage Retinal hemorrhage Retinopathy Eclampsia Hypertensive encephalopathy Microangiopathic hemolytic anemia Acute kidney injury Aortic dissection / aneurysm rupture Hypertensive Emergency: Systolic BP > 180 mmHg or diastolic BP >110 mmHg with evidence of end organ damage. Treatment Goals: Initial reduction of 20-25% in SBP within the first 1-2 hours ; once achieved, further reduction to a target of 160/100 in 6-12 hours then a return to a normal blood pressure in 24-48 hours. The primary difference in hypertensive urgency and emergency is the rapidity of rise. Hypertensive emergency is accordingly associated with diffuse necortizing vasculitis, arteriolar thrombi and fibrin deposition in arteriolar walls whereas urgency is not. Walmart / Target $4 Formulary Beta Blockers Atenolol Carvedilol Metoprolol Tartrate Nadolol Pindolol Ace Inhbitors Benazepril† Enalapril* Lisinopril* Thiazide Diuretics Chlorthalidone HCTZ* Loop Diuretics Bumetaide Furosemide Vasodilators Diltiazem Hydralazine Verapamil (25, 50, 100) (3.125, 6.25, 12.5, 25) (25, 50, 100) (20, 40) (5, 10) (5, 10, 20, 40) (2.5, 5, 10, 20) (2.5, 5, 10, 20) (25, 50) (12.5, 25, 50) (0.5, 1) (20, 40, 80) (30, 60, 90, 120) (10, 25) (80, 120) *Widely available fixed dose combinations with HCTZ †Widely available fixed dose combinations with amlodipine Antihypertensive Regimen Choice: When starting patients on an antihypertensive regimen (including diabetic patients), begin with either a thiazide diuretic or calcium channel blocker (Amlodipine or Nifedipine). As a general principle, try to stick to HCTZ or Amlodipine (amlodipine is also an anti-anginal) as both are widely available and come in a wide range of two drug combinations. If a second agent is needed, start an ACE or ARB. Always remain coginizant of how many times a day the regimen you prescribe needs to be taken (e.g., Hydralazine TID). When possible, look into switching to fixed dose combinations as there is some data to suggest that compliance improves with these formulations. Otherwise the non-compliance rate is generally >30%. EKG INTERPRETATION Reading an EKG may seem overwhelming at first but the following instructions are to help guide your evaluation. You should approach an EKG systemically every time, making the potential abundance of information more manageable. Approach them in the following order, even if it appears to be straightforward. 1. Rate. For a regular rhythm, count the large boxes between two QRS complexes. 1 small box = 0.04 s For an irregular or severely bradycardic rhythm, count the number of QRS complexes on the full 12 lead EKG strip and multiplying by 10 yielding the average beats per minute. 300 150 100 75 60 50 1 large box = 0.20 s 2. Origin of Rhythm. Assess for the presence of a P-wave before every QRS, and upright P-waves in leads I and II – if present, the rhythm is likely normal sinus (60 - 100 bpm). Inverted or abnormal P-waves suggest an ectopic atrial rhythm (P:QRS is1:1). SA Node 300 150 100 75 60 50 AV Node No P-wave with a narrow regular QRS complex suggests a junctional rhythm (40 - 60 bpm vs accelerated 60-100 bpm). Ventricular Escape 300 150 100 75 60 50 43 No P-wave with a wide QRS complex suggests a ventricular escape rhythm (20-40 bpm). 300 150 100 75 60 50 43 EKG INTERPRETATION 3. Axis. A normal axis is positive in leads I, II and aVF. Determine the quadrant based upon the orientation of leads I and aVF (to be more precise, then find which lead is most isoelectric). Rig h aVR / 120° I aVF -30° / II 0° / aVF I / 90° I isolectric / -90° -60° / aVR Left Ax Nor ma II / 150° aVF Extr em xis tA aVF / 180° I e ht Rig is aVF I / -90° aVL / -120° aVF III / -150° No r I Left Ax xis tA ht Rig l ma Extr em e is aVF Step 2: Find lead where QRS is most isoeletric. I Rig h I l Step 1: Quadrant 30° / III 60° / aVL Left Axis Deviation (-30° to -90°): - LVH, inferior MI, WPW, ostium secundum ASD - Left anterior fascicular block (-45° to -90°): qR in aVL, no other cause -30° II isolectric of left axis deviation. QRS <100 ms unless aberrant conduction present. You cannot code a LAFB if you have an inferior MI. Right Axis Deviation (90° to 180°): aVF I isolectric - RVH, PE, COPD, lateral MI, WPW, ostium primum ASD 180° - Left posterior fascicular block: rS in I and aVL and qR in III and aVF, no other causes of R axis deviation - QRS narrow unless aberrant conduction present. 4. Intervals. RR Interval ST Interval QT Interval PR I I isolectric / 90° aVF EKG INTERPRETATION 4. Intervals. PR Interval (normal 120 - 200 ms): - Short PR (<120 ms): Wolff-Parkinson-White, AV nodal rhythm, low atrial ectopic rhythms. - Long PR (>200 ms): 1° aV block, higher degree heart block, hypokalemia, rheumatic fever, Lyme disease. QRS Interval [duration] (normal 60 - 100 ms): - Narrow QRS (<60 ms): Rarely seen. Hypocalcemia. - Wide QRS (>120 ms): Bundle branch blocks / nonspecific conduction delays, VT / VF, hyperkalemia, accessory pathways with preexcitation, ventricular escape rhythms. QT Interval (normal <450 ms): Varies with heart rate (QTc ). More concerning when QTc >500 ms. - Prolonged QTc (> 450 ms): Medications (see www.qtdrugs.org), hypocalcemia, hypokalemia, hypomagnesaemia, ICH, stroke, carotid endarterectomy, neck dissection, congenital long QT (may not be present on resting EKG), K/Na/Ca channelopathies, CAD, cardiomyopathy, hypothyroidism, hypothermia. 5. Atrioventricular Blocks. 1° AV Block (Physiologic): PR > 200 ms and P to QRS is 1:1. - No treatment necessary if seen in isolation 2° AV Block Type I (Wenckebach) (Physiologic): Progressive lengthening of PR interval until impulse not conducted, exhibits “grouped beating.” - No treatment necessary unless severely bradycardic or symptomatic 2° AV Block Type II (Mobitz) (Pathologic): Ocasional or repeatedly non-conducted impulses with consistent PR interval. Level of the block is typically infrahisian. - Requires pacemaker, often worsens to third degree 3° AV Block (Pathologic): Complete AV dissociation, irregular PR intervals, P waves and QRS complexes are both regular but indepentent of one another. - Morphology of QRS complex dependent on origin of escape rhythm - Requires pacemaker EKG INTERPRETATION 5. Hypertrophy and Voltage. Atrial Enlargement. II RA Enlargement >2.5 mm in II, III & aVF >1.5 mm in V1 or V2 II V1 Normal II LA Enlargement Terminal portion in V1 >1 deep & 1 wide Notched P > 120 ms in II, III or aVF Ventricular Hypertrophy and Low Voltage. Low Voltage: Requires < 10 mm in all precordial leads and < 5 mm in all limbs. Seen with chronic lung disease ; pericardial / pleural effusions ; obesity ; cardiomyopathies ; CAD with extensive LV infarction ; myxedema Left Ventricular Hypertrophy Cornell: R in aVL + S in V3 greater than 28 mm in men / 20 mm in women Alternate criteria for precordial and limb leads (one or more): 1) R in V5 or V6 + S in V1 > 35 mm (40 yrs) ; > 40 mm (30-40 yrs) 2) Maximum R wave + S wave in precordial leads > 45 mm 3) R wave in V5 > 26 mm ; in V6 > 20 mm 4) R wave in I + S in II > 25 mm ; R in I > 14 mm ; aVL > 12 mm ; aVF > 21 mm Right Ventricular Hypertrophy Right axis deviation with axis > 100°, downsloping ST depression and T inversions in right precordial leads and one of the following 1) R / S ratio in V1 >1 or R / S ratio in V5 or V6 < 1 2) R in V1 > 7 mm 3) rSR’ in V1 with R’ > 10 mm 6. R Wave Progression. R wave amplitude should increase with the progression of the precordial leads assuming appropriate placement and should be > 3 mm by V3. Poor progression may be caused by anteroseptal MI, LVH, dilated cardiomyopathy. 7. Q Waves. Q Waves: <30 ms common but all Q waves in V1-3 and any in I, II, aVL, aVF and V4-6 lasting over 30 ms are abnormal. For infarct identification, Q waves must be seen in 2 or more contiguous leads. Isolated Q waves in lead III are not uncommon and do not carry any known prognostic significance. EKG INTERPRETATION 8. Bundle Branch Blocks. Left Bundle Branch Block Right Bundle Branch Block QRS Duration must be > 120 ms (incomplete if QRS is >100 ms but otherwise appears like a LBBB) I QRS Duration must be > 120 ms (incomplete if QRS is >90 ms but otherwise appears like a RBBB) V6 V1 I Lead 1: Monophasic R & no Q waves. Lead V1: QS or rS pattern Lead V6: Late intrinsicoid deflection, Monophasic R & no Q waves. V1 V6 Lead 1: Wide S wave. Lead V1: Late intrinsicoid deflection. M-shaped QRS (RSR’). Sometimes wide R or qR Lead V6: Early intrinsicoid deflection with a wide S wave. 9. ST Segment Changes. ST Segment Identification: Starting 0.06 s after J point and measure in mm relative to TP segment. J point Normal Variation Normal ST Segments: Usually isoelectric but may vary from 0.5 mm depression to 1mm elevation in limb leads and up to 3 mm concave upward elevation in the precordial leads in early repolarization. ST Segment Diagnosing MI with LBBB (Sgarbossa’s Criteria): Scores ≥ 3 are 80% sensitive and 90% specific for AMI 3 QRS Axis ≥ 1 mm ≥ 5 mm ≥ 1 mm QRS Axis 2 QRS Axis 1 5 Points 2 Points 3 Points ST elevation ≥ 1 mm concordant with QRS in any lead. ST elevation ≥ 5 mm disconcordant with QRS in any lead. ST depression ≥ 1 mm in V1, V2 or V3. 10. Infarct Localization. I aVR V1 V4 II aVL V2 V5 III aVF V3 V6 Inferior (PDA) Anterior (LAD) Lateral (Circ) Septal (LAD) AVNRT vs AVRT AV Nodal Reentrant Tachycardia (AVNRT): Initiated with a premature complex (PAC / PVC) and can be divided into one of two varieties: 1) typical AVNRT (antegrade conduction down slow pathand retrograde up fast path): 80-90% of cases and 2) atypical AVNRT (antegrade down fast path and retrograde up slow path): 10-20% of cases. Rhythm is rapid and regular with normal QRS duration unless there is co-existing conduction system disease (RBBB / LBBB or a rate dependent bundle branch block). Sinus impulse conducted down fast pathway (long refractory period). PAC occuring while fast path still refractory and conducts down slow path. SA Node Impulse from PAC enters ventricles and finds fast pathway reset, travels retrograde up fast pathway and finds slow pathway reset and creates a loop. PAC Refractory Slow Termination of AVNRT via Adenosine blockade of AV node (or vagal maneuvers or DCCV). May terminate in either a ventricular or atrial complex. Adenosine Block Fast PAC Retrograde P AV Reentrant Tachycardia (AVRT): Initiated with a premature atrial complex (PAC) and can be devided into one of two varieties: 1) typical / orthodromic AVRT (antegrade conduction down fast path and retrograde up accessory path (narrow QRS) 95% of cases and 2) atypical (antidromic) AVRT antegrade down accessory path and retrograde up AV node (wide QRS) 5% of cases. Sinus impulse conducted down fast path and accessory path. PAC travels down AV node, through ventricles and retrograde up accessory pathway establishing loop (orthodromic / typical AVRT). PAC SA Node Slow PAC Fast Accessory Pathway Adenosine Block Orthodromic Orthodromic Antidromic Termination of Orthodromic AVRT via Adenosine blockade of AV node (or vagal maneuvers or DCCV) terminates in an atrial complex. Antidromic AVRT terminates in a ventricular complex. Antidromic PAC Retrograde P Orthodromic Adenosine Block Antidromic ATRIAL TACHYCARDIAS Atrial Tachycardia: Broad term used to describe a complex of atrial tachyarrhythmias including atrial flutter. It encopmasses a discrete atrial ectopic focus driving a tachyarrhythmia and re-entrant tachycardias like atrial flutter. Sinus Beat Focal A Tach Sinus Beat 2:1 A Tach Multifocal Atrial Tachycardia: Rare arrhythmia. Halmark is the identification of 3+ ectoptic atrial foci driving the tachyarrhtyhmia. Treated in a similar fashion as typical focal atrial tachycardia. Sinus Beat Multifocal A Tach P P1 P2 P1 P2 P3 P3 P1 P2 P2 P1 Atrial Flutter: Atrial flutter rate 240 - 350 with the ventricular rate dependent upon AV conduction (may be conducting in a set ratio such as 1:4 or have variable AV conduction). Typical atrial flutter has sawtooth pattern with negative flutter waves in the inferior leads and positive flutter waves in V1. Atrial Fibrillation: Seemingly chaotic atrial activity (mechanism is a matter of debate). Ventricular repsonse typically very irregular but may seem regular if very slow (<70 bpm) which is also indicative of significant conduction system disease. Do not expect to alway see a fibrillating baseline. CHA2DS2-VASc Scoring System Heart Failure / L V dysfunction Hypertension Age 65-74 75+ Diabetes Mellitus Stroke / TIA PAD / Old MI / Aortic Plaque Female CHA2DS2-VASc Annual Stroke Risk 1 1 1 2 1 2 1 1 Lip et. al. Chest. 2010;137(2):263-272 0 1 2 3 4 5 6 7 8 9 1.3% 2.2% 3.2% 4.0% 6.7% 9.8% 9.6% 6.7% 15.2% BRADYCARDIAS Before attempting to treat a patient for symptomatic bradycardia it is imperative that you understand the pathology driving their bradycardia as medications such as Isoproterenol, Atropine and Epinepherine can paradoxically worsen high grade heart block. SA and AV Nodally Mediated Bradycardias 1 2 3 1 Pure sinus bradycardia driven by poor intrinsic SA nodal activity or by excess vagal tone (or drug effect). Stimulation with Isoroterenol or Epinepherine or disinhibition with Atropine will raise SA nodal rate. 1 Sinus bradycardia driven by excess vagal tone (e.g. untreated OSA). Progressive RR prolongation before potentially very long sinus pauses (sometimes 6+ seconds) while sleeping. Stimulation with Isoroterenol or Epinepherine or disinhibition with Atropine will raise SA nodal rate and improve AV nodal conduction, but typically the goal is to fix the underlying problem (e.g. CPAP). 2 Second Degree AV Block type I (Wenckebach) driven by intrinsic AV nodal delays, typically physiologic but may also be a manifestation of nodal ischemia or valvular disease. Stimulation with Isproterenol or Epinepherine or disinhibition with Atropine will typically improve AV nodal conduction but this is rarely needed. 2 Atrial Fibrillation with Slow Ventricular Response. Slow ventricular rates a manifestation of either high frequency stimulation of the AV node with and longer refractory periods and or of infranodal disease. Stimulation with Isproterenol or Epinepherine or disinhibition with Atropine will probably not have any significant effect or may worsen the ventricular response, especially if there is concurrent bundle branch blocks. 2 Complete Heart Block. Slow ventricular rate a manifestation of the secondary pacemaker site (narrow QRS junctional, wide QRS may be fascicular or ventricular). Stimulation with Isproterenol or Epinepherine or disinhibition with Atropine may accelerate the secondary pacemaker but if the patient is in any way unstable should have transcutaneous pacing preferentially. Infranodal and Intra-His Mediated Bradycardias 3 Atrial Fibrillation with Slow Ventricular Response and RBBB. Slow ventricular rates likely a manifestation of high frequency stimulation of the AV node compounded by concurrent infranodal disease with hyperpolarization of the his bundles causing longer refractory periods. Stimulation with Isproterenol or Epinepherine or disinhibition with Atropine may worsen the bradycardic response by augmenting the his bundle hyperpolarization and refractoriness. 3 Atropine or Isoproterenol Second Degree AV Block type II (Mobitz) driven by infrahisian conductoin system disese. Stimulation with Isproterenol or Epinepherine or disinhibition with Atropine may worsen the bradycardic response by augmenting the his bundle hyperpolarization and refractoriness. WIDE COMPLEX TACHYCARDIA Wide QRS Complex Tachycardia QRS > 120 ms Regular R-R Irregular R-R Atrial fibrillation / flutter / tachycardia with abberent conduction (BBB, IVCD or accessory pathway) QRS identical to resting EKG SVT, RBBB / LBBB and antidromic AVRT Vagal Maneuvers Adenosine IVP QRS differs from resting EKG and prior MI or structural disease Likely VT 1:1 AV Ratio or Unknown Concordant precordial leads No RS pattern Onset of R to nadir > 100 ms Ventricular Tachycardia RBBB Patern qR, Rs or Rr’ in V1 Frontal axis +90° to -90° < 1:1 AV Ratio > 1:1 AV Ratio Likely VT Atrial tachycardia / flutter R to S > 100 ms V1 V2 V3 V6 I II Left Ventricular Tachycardia LBBB Patern R in V1 > 30 ms R to nadir of S in V1 > 60 ms qR or qS in V6 aVF I II Right Ventricular Tachycardia American Heart V5 V4 Association Blomström-Lundqvist et al. JACC. 2003;42:1493–531 aVF V1 V6 V1 V6 CHEST PAIN EVALUATION Definitions of angina: Typical Angina (Definite): Substernal chest pain or discomfort that is 1) provoked by exertion or emotional stress and 2) relieved by rest and/or nitroglycerin. Atypical Angina (Probable): Chest pain or discomfort that lacks one of the characteristics of definite or typical angina. Nonanginal Chest Pain: Chest pain or discomfort that meets one or none of the typical angina characteristics. Diamond and Forrester Pre-Test Probability of Coronary Artery Disease Age (Years) Sex Typical Angina Atypical Angina Nonanginal Chest Pain <40 Man 10-90% 10-90% <10% Woman 10-90% <5% <5% 40-49 Man >90% 10-90% 10-90% Woman 10-90% <10% <5% 50-59 Man >90% 10-90% 10-90% Woman 10-90% 10-90% <10% Man >90% 10-90% 10-90% Woman >90% 10-90% 10-90% >60 ELECTROCARDIOGRAPHY - LEAD PLACEMENT V1 V2 V3 V4 V5 V6 V4r V5r V6r 4th right intercostal space 4th left intercostal space Directly between V2 and V4 5th left ICS at the MCL 5th left ICS at ant ax line 5th left ICS at mid ax line 5th right ICS at the MCL 5th right ICS at ant ax line 5th right ICS at mid ax line RA LA LL RL Right Arm Left Arm Left Leg Right Leg ACUTE CORONARY SYNDROME Algorithm for the evaluation and management of patients suspected of having ACS SYMPTOMS SUGGETIVE OF ACS Possible ACS Definite ACS ST elevation No ST elevation Activate STEMI Pager Nondiagnostic ECG Normal initial troponin ST and/or T wave changes Ongoing pain Positive troponin Hemodynamic abnormalities Observe Follow-up at 4-8 hours: ECG , troponin No recurrent pain; Negative follow-up studies Stress study to provoke ischemia Consider evaluation of LV function if ischemia is present (Tests may be performed either prior to discharge or as outpatient) Negative Potential diagnoses: nonischemic discomfort; low-risk ACS Recurrent ischemic pain or Positive followup studies Diagnosis of ACS confirmed Positive Diagnosis of ACS confirmed Arrangements for outpatient follow-up American Heart Association Eugene Braunwald et al. Circulation. 2000;102:1193-1209 Admit to hospital Manage via acute ischemia pathway ACUTE ISCHEMIA PATHWAY Recurrent ischemia and / or ST segment shift, or Deep T-wave inversion, or Positive troponin Aspirin Beta blockers Nitrates Antithrombin Regimen GP IIb/IIIa inhibitor Monitoring (rhythm and ischemia) Early invasive strategy Immediate angiography 12-24 hour angiography Early conservative strategy Recurrent symptoms/ischemia Heart failure Serious arrhythmia Patient stabilizes Evaluate LV Function LVEF <40% LVEF >40% Stress Test Not low risk Low risk Follow on medical Rx American Heart Association Eugene Braunwald et al. Circulation. 2000;102:1193-1209 ACUTE CORONARY SYNDROME Not all troponin leaks are secondary to acute myocardial infarction. THINK is the TROPONIN ELEVATION due to PLAQUE RUPTURE or secondary to another underlying cause. Supply demand imbalance alone Vasospasm or endothelial dysfunction Type 2 Myocardial Infarction (Demand Ischemia) Fixed atherosclerosis and supply demand imbalance Plaque rupture with thrombus Type 1 Myocardial Infarction STEMI: Clinical syndrome defined by 1) symptoms of myocardial ischemia in association with 2) persistent ECG ST elevation and 3) subsequent release of biomarkers of myocardial necrosis. 1) New ST elevation at the J point in at least 2 contiguous leads of 2+ mm in men or 1.5+ mm in women in leads V2–V3 and or of 1 mm in other contiguous chest leads or the limb leads. 2) New or presumably new LBBB 3) ST depression in 2 precordial leads (V1–V4) may indicate transmural posterior injury 4) Multilead ST depression with coexistent ST elevation in lead aVR has been described in patients with left main or proximal left anterior descending artery occlusion. 5) Hyperacute T-wave changes may be observed in the very early phase of STEMI, before the development of ST elevation NSTEMI: Clinical syndrome defined by 1) symptoms, 2) absence of persistent ST elevation but can have other ST-T wave changes, and 3) release of cardiac biomarkers (2 of 3 criteria must be met). Unstable Angina: Clinical syndrome defined by 1) symptoms, 2) absence of persistent ST elevation but can have other ST-T wave changes, and 3) release of cardiac biomarkers. x Upper Limit of Normal 100 Troponin without reperfusion Troponin with reperfusion CKMB without reperfusion CKMB with reperfusion 20 10 5 2 1 0 0 1 2 3 4 5 Days from Onset of Infarction 6 7 8 ACUTE CORONARY SYNDROME TIMI Risk Score (NSTEMI) Composite Risk (%) 50 40 30 20 10 0 0-1 2 3 4 5 6-7 Indicator of 35 day composite events risk (mortality, new or repeat MI, severe recurrent ischemia requiring urgent revascularization through 14 days after admission). 1) 2) 3) 4) 5) 6) 7) Age >65 years 3+ cardiac risk factors Prior coronary stenosis >50% ST segment deviation on admission ECG >2 anginal events in the last 24 hours Aspirin treatment in the prior 7 days Prior congestive heart failure, MI, CABG or PCI Medications to be started immediately (these have survival benefits) to be administered to all patients (unless clear contraindication). 1. Aspirin 325 mg PO once followed by Aspirin 81 mg PO daily 2. Heparin drip (intermediate algorithm with bolus) 3. Lipitor 40-80 mg PO QHS 4. Plavix / Effient / Brilinta (P2Y12 inhibitors) should not be started unless told to do so explicitly by the cardiology fellow or attending. If started before you know if the patient will need CABG it may delay surgery for a week. If they are already taking one of these agents however`, they should be continued. Medications to be started before discharge (these have survival benefit but do not need to be started immediately). 5. Beta blocker (typically Metoprolol or Carvedilol) 6. Ace inhibitor / ARB if LVEF <40%, comorbid hypertension, diabetes or CKD Medications for symptomatic relief or minimal if any survival benefit. 7. Nitroglycerin 0.3 - 0.4 mg SL Q5min x 3 PRN for continuing angina and consider starting IV nitroglycerin. 8. Supplemental oxygen only if SaO2 <90% or respiratory distress Ancillary testing to be obtained on all patients admitted with an acute coronary syndrome: 1) Lipid panel 2) Hemoglobin A1c 3) Transthoracic echocardiogram (with definity if a large anterior wall MI or concern for aneurysm formation which could predispose to LV thrombus formation. CARDIAC CATHETERIZATION Routine cardiac catheterizations are performed Monday through Friday (7:00 am to 5:00 pm) urgent and emergent cases are performed after hours. Cases typically performed with local anaesthesia and light conscious sedation (Versed and Fentanyl). Before requesting a procedure be done for a patient, there must be a reasonable risk benefit assessment and the expectation that the information or therapeutics gleaned from the study will offset the risks of the procedure. Preprocedure Checklist Contraindications EKG CBC INR > 2 Hemodynamically Unstable INR NPO Platelets < 50 Active Hemorrhage BMP Consent AKI (Angiogram) Stroke / CNS Bleed Pericardiocentesis (30 min): Effusion must be accessible by a subcostal or apical approach. Right heart catheterization (10-15 min): With shunt run this becomes much longer, usually around 45 minutes to 1 hour. Left heart catheterization (30 - 60 min): If done as part of an aortic valve study (with or without dobutamine) it will add roughtly another 30 min. Stenting can usually be done at the same time as the angiogram but the time it takes to complete varies greatly with complexity of the intervention. Balloon Pump Placement (15 - 45 min): If removing a prior balloon pump, add another 30 minutes to the procedure to achieve hemostasis. Common Angiographic Views Left Main RCA Left Main Left Main Circumflex Circumflex RCA LAD LAD RCA Circumflex LAD PDA PDA Images courtesy of Patrick J. Lynch PDA VALVULAR HEART DISEASE St. Jude Mechanical Medtronic Mosaic Bovine Pericardial Medtronic CoreValve Evolut Image courtesy of Patrick J. Lynch American Society for Echocardiography Reference Ranges Aortic Stenosis Velocity (m/s) Mean Pressure Gradient (mmHg) Valve Area Aortic Regurgitation Vena Contracta (cm) Mitral Stenosis Planimetry MV Area (cm2) DIastolic Pressure Half Time (ms) Mean Pressure Gradient (mmHg) Mitral Regurgitation Vena Contracta (cm) ERO (cm2) Normal Mild Moderate Severe <2 <10 >2 2.0 - 2.9 <20 >1.5 3.0 - 3.9 20 - 39 1.0 - 1.5 >4.0 >40 <1.0 <0.3 0.3 - 0.6 >0.6 >1.5 <150 <5 1.0 - 1.5 >150 5 - 10 <1.0 >220 >10 <0.3 American Heart AssociationNishimura et. al. J Am Coll Cardiol. 2014;63(22):e57-e185 <0.7 <0.4 >0.7 >0.4 CARDIAC DEVICES Device Program Coding Standard coding involves three letters. 1st denotes which chambers are paced. 2nd denotes which chambers are monitored for intrinsic activation. 3rd denotes the response to a native activation. Pulse Generator Most common modes: DDD - Paces both RA and RV (sometimes BiV). Monitors RA and RV. Detections may trigger a pacing impulse or inhibit depending upon their timing. Coronary Sinus Lead (CS) (Pace / Sense) Right Atrial Lead (RA) VVI - Paces RV. Monitors RV. Detections inhibit pacing. (Pace / Sense) Right Ventricular Lead (RV) (Pace / Sense / Defibrillate) AAI - Paces RA. Monitors RA. Detections inhibit pacing. AAI <-> DDD - Converts between modes when in AFib or Flutter. PACEMAKERS DEFIBRILLATORS Designed to treat bradyarrhythmias. They only pace when the patient’s intrinsic heart rate is less than the programmed lower rate limit. They do not treat tachyarrhythmias. Designed to treat tachyarrhythmias (primarily VT / VF) most defibrillators also have the ability to treat bradyarrhythmias via a pacing function (with the exception of subcutaneous ICDs which only defibrillate). Device Type Leads Sensing RA RV CS Single Chamber Pacemaker Dual Chamber Pacemaker Biventricular Pacemaker (CRT-P) Single Chamber Defibrillator (ICD) Dual Chamber Defibrillator (ICD) Biventricular Defibrillator (CRT-D) Subcutaneous ICD (SC-ICD) Therapies Pacing ATP RV RV RV & RA RV & RA RA / RV / CS RA / BiV RV RV RV & RA RV & RA RA / RV / CS RA / BiV SC Coil Shock EP PROCEDURES Routine electrophysiology procedures (device implantations and ablations) are performed Monday through Friday (7:00 am to 5:00 pm) urgent and emergent cases are performed after hours. Pacemakers are often performed with local anaesthesia and conscious sedation (Versed and Fentanyl) but more complex procedures will typically be performed with anaesthesia. Before consulting the electrophysiology service there must be a risk benefit assessment and a reasonable expectation of quality and life and expected survival of at least one year from any device implantation or upgrade. Similarly if the patient has bradycardia or other arrhythmias due to reversible causes or remediable problems such as electrolye derrangements, sleep apnea or digoxin toxicity, they are generally not appropriate candiates for device implantation. Preprocedure Checklist Type and Screen CBC NOACs held as directed INR NPO Coumadin OK to continue BMP Heparins stopped at least 12 hours earlier Single or Dual Chamber Pacemaker Implantation (60 min): Indicated for 1) symptomatic bradycardia or chronotropic incompetence, 2) high grade heart block, 3) alternating bundle branch blocks, 4) sinus pauses while awake of >3 seconds and 5) atrial fibrillation conversion pauses while awake of >5 seconds. Cardiac Resynchronization Therapy (CRT-P / CRT-D) (1 - 3 hours): Indicated for patients with an LVEF <35% and a QRS duration >120 ms in sinus rhythm with class III or ambulatory class IV heart failure despite optimal medical therapy. Defibrillator Implantation (60 min): Indicated for 1) patients who survived a cardiac arrest from VF / VT or sustained VT after exclusion of reversible causes and 2) patients with an LVEF <35% (and at least 40 days post MI if their heart failure is ischemic in nature) and are in NYHA class I-III heart failure. Atrial Flutter Ablation (1 - 2 hours): Indicated as front line therapy for treatment of typical atrial flutter. If onset of atrial flutter is > 48 hours before the procedure or the patient has not been on anticoagulation, they will usually require a TEE first. Atrial Fibrillation Ablation (4 - 6 hours): Typically this is a very complicated procedure and generally only done as an outpatient. Ventricular Tachycardia Ablation (4 - 8 hours): Potentially a very complicated procedure and given the length of the procedure usually requires 2-3+ L of IVF being given to prevent thermal burns from the ablation catheters (require continuous flushing). Post Device Implantation Checklist Do NOT restart any heparins or NOACs (this includes DVT prophylaxis) for 1-2 weeks Coumadin OK to continue Chest XR ( PA / Lateral) in AM Site dressings managed by EP Service Device interrogation in AM HEART FAILURE Volume Status vs Perfusion: Measure JVD in CM above sternal notch (measured + 5). Normal is 7-9 Volume status: Jugular vein distention (JVD), hepatojugular reflux, peripheral edema, orthopnea, PND, rales. Perfusion: Look for evidence of end organ hypoperfusion (cool extremities, renal failure, acidosis, altered mental status etc...). 45 Degrees It is extremely important to remember that these are not problems occuring in isolation and in most patients, there is a mix of both problems at any one given time. ACC / AHA Stage NYHA Stage A High risk for heart failure but without structural heart disease or symptoms. B Structural heart disease without heart failure. I C Structural heart disease with prior or active heart failure. II III Symptoms with moderate exertion D Refractory heart failure requring specialized interventions. IV Symptoms at rest 1 Year Mortality Based on comorbidities Asymptomatic Symptoms with minimal exertion 5-10% 15-30% 50-60% Documentation: Your note must stipulate acute, chronic or acute on chronic systolic or diastolic heart failure. Terms such as HFpEF and HFrEF are not acceptable. After establishing the etiology (if known), you need to note the ACC and NYHA stage as detailed above. Initial Management: Once you identify the major problem (volume overload and or poor stroke volume) tailor treatment accordingly (diuretics or dialysis for volume overload and inotropes, balloon pumps and LVADs for poor LV function). Right Heart Catheterization: The use of pulmonary artery catheters to guide heart failure managment was formally evaluated in the ESCAPE trial (PMID 16204662). This study failed to show any benefit to the use of pulmonary artery catheters to guide therapy over just clinical assessment of Diuretic Equivalence volume status. There is Agent Onset Duration Bioavailability currently no well agreed Lasix (40 mg PO) 30-60 min 6-8 hrs 50% indication for pulmonary artery Lasix (20 mg IV) 5 min 2 hrs 100% catheter placement to guide Bumex (1 mg PO) 10 min 4-6 hrs 80% therapy outside of documentDemedex (10 mg PO) 1 hr 4-6 hrs 90% ing inotrope dependence. Metolazone (PO) 60 min >24 hrs HEMODYNAMICS ECG RA a c v x 25 y 0 mmHg RV Tricuspid Valve Closes 25 Tricuspid Valve Opens 0 mmHg Reference Ranges Right Atrium a wave v wave Mean 2 - 7 mmHg 2 - 7 mmHg 1 - 5 mmHg Right Ventricle Systolic EDP 15 - 30 mmHg 1 - 7 mmHg Pulmonary Artery Systolic Diastolic Mean 15 - 30 mmHg 4 - 12 mmHg 9 - 19 mmHg 25 Pulmonic Valve 0 mmHg Closes a c PCWP v x 0 mmHg 125 Aortic Valve Opens Aortic Valve Closes Ao 90 - 140 mmHg 60 - 90 mmHg 70 - 105 mmHg Cardiac Index > 2.5 L/min/m2 100 75 90 - 140 mmHg 5 - 12 mmHg Aortic Systolic Diastolic Mean 25 y Pulmonary Capillary Wedge Mean 4 - 12 mmHg Left Ventricle Systolic EDP PA Pulmonic Valve Opens 50 Mitral Valve Closes Mitral Valve Opens LV edp 25 0 mmHg IABP B A E 125 Aortic Valve Opens Pres s Ao On Swan Daily Rounds Nativ e P IAB ith W C ures Aortic Valve Closes Chest XR for catheter position Assessment of insertion site Systole Diastole D Systole 100 75 50 mmHg BALLOON PUMPS PA IAB Coronary Perfusion Augmentation Systole Diastole Systole Image redrawn from Jones et. al. J Invasive Cardiol 2012; 24:544-550 ti ta en m ug Nativ e 125 Pres sures 100 on 75 50 mmHg Diastole Benefit is garnered by a reduction in the work required by the LV during systole (balloon deflates providing a partial vacuum into the aorta) and an increase in the diastolic pressure (during coronary perfusion). All Cause Mortality from SHOCK II Trial (IABP Support in ACS with Cardiogenic Shock) 60 IABP Control Mortality (%) 50 40 30 20 10 0 p=0·94; log-rank test Relative risk 1·02, 95% CI 0·88–1·19 0 30 60 90 120 150 180 210 240 270 Days after randomisation 300 330 360 390 420 The use of intraaortic balloon pumps for the treatment of acute cardiogenic shock has mostly been studied in cases of an acute coronary syndrome (such was the subject of the SHOCK II trial). They are also commonly used in cases of cardiogenic shock from other etiologies such as fulminant myocarditis or acute on chronic systolic heart failure. In ACS patients their use has failed to show benefit as shown above. In the other situations, there is little if any data to assess their value. Balloon Pump (IABP) Daily Rounds Chest XR to confirm IABP position Most recent aPTT and trend Assessment of IABP insertion site Assessment of pedal pulses and perfusion Balloon Pump (IABP) Anticoagulation Heparin drip (low dose nomogram) Proper placement of IABP with radio-opaque marker just below aortic arch and about the level of the carina. LEFT VENTRICULAR ASSIST DEVICES Two models in use are the Heartmate II and HeartWare. Both have a mechanical pump which draws blood from the LV cavity at the apex and shunts it to the aortic root. This augments the native LV function and there is usually a pulse during systole. Occasionally, the residual LV function is so poor that there is no palpable pulse and the aortic valve does not open. To measure blood pressure in these patients use a manual cuff and a doppler probe, record the first audible sound during deflation as the MAP (goal is 70-80 mmHg). Reported Pump Parameters: Pump Speed (Set) Power (Measured) Flow (Estimated) Pulse Index (Estimated) Flow (L/min) 8 Inlet (LV) Pressure Outlet (Aortic) Pressure Pump Flow 120 6 90 4 60 2 30 0 0 Characteristic Electrical Baseline EKG Intereference Pressure (mmHg) LVAD Flow and Pressure L/min 2920 RPM 4.2 Watts Power (Watts) 4.3 12 Flow (L/min) HEARTWARE LVAD (HVAD) 12 Fixed 4 POD: 8 1 9 8 7 6 5 4 Time (s) 3 2 1 9 8 7 6 5 4 Time (s) 3 2 1 8 4 Time Scale Sx Off HW1234567 22:43:56 8 HeartWare 2 Reprinted with the permission of HeartWare © HeartWare HVAD Function Reference Ranges Set Speed (RPM) 1800 2400 INR Patient System Controller Display (Attached via Driveline) Alarm Mute 2.5 8.5 1.8 3 8 10 1 2 3 4 Power (Watts) Flow (L/min) 3200 4000 Battery Indicator 1 Alarm Indicator Battery Indicator 2 Scroll 3000 RPM 5.0 L/min 4.8 Watts HeartWare Critical Alarms HeartWare HVAD Daily Rounds Confirmation of pump speed INR Pump flow and power trends NO MESSAGE - No power to pump / Pump has stopped Steady Tone Flashing Red Hgb, PLT and LDH trends Arrhythmia review Suction event review HeartWare HVAD Anticoagulation Aspirin 325 mg PO QD Coumadin (INR 2-3) Two Tone VAD STOPPED - Driveline disconnected, fracture or connector malfunction. VAD electrical failure, Controller / VAD Failure. Thrombus or other material in device. CONTROLLER FAILED - Controller Component Failed CRITICAL BATTERY - Limited battery life remaining or malfunction. The nursing staff has been trained to care for most of these scenarios. In the event of repeated alarms, notify the on call fellow immediately. HEARTMATE LVAD (LVAD) Clinical Settings Pump Flow Alarms Save Data History Pump Speed 4.5 9500 lpm Admin Pulse Index rpm Display ON/OFF Fixed Mode - Speed Setpoint: 9600 rpm 3.6 Pump Power 5.7 w Reprinted with the permission of Thoratec Corporation © LVAD Functional Reference Ranges Set Speed (RPM) 6K 8K 10K 15K 2.5 10 2.5 3 10 1 2 3 Power (Watts) Flow (L/min) INR Patient System Controller Display (Attached via Driveline) Test Select Button Battery Fuel Gauge Battery Symbol (yellow & red) 4 Power Symbol Controller Cell Symbol Red Heart Symbol Silence Alarm Button Critical Alarms HeartMate II LVAD Daily Rounds Red Heart Confirmation of pump speed INR Pump flow, PI and power trends Steady Tone Red Battery Hgb, PLT and LDH trends Arrhythmia review PI / Suction event review Steady Tone Yellow Battery HeartMate II LVAD Anticoagulation Aspirin 325 mg PO QD Coumadin (INR 2-3) Pump flow < 2.5 Lpm, pump has stopped, perc lead is disconnected, or pump is not working properly. 1 Beep Q4 seconds < 5 min battery power remains, voltage too low, or the System Controller is not getting enough power. < 15 min battery power remains, voltage too low, or the System Controller is not getting enough power. The nursing staff has been trained to care for most of these scenarios. In the event of repeated alarms, notify the on call fellow immediately. PREOPERATIVE EVALUATION Urgency Emergent Definition Serious threat to life or limb if not in the operating room within <6 hours. *Provide risk stratification but the patient should proceed directly to surgery without delay. Urgent Serious threat to life or limb if not in the operating room within 6-24 hours. Time Sensitive No immediate threat to life or limb but excess delay for clinical evaluation (>6 weeks) will negatively effect outcome. Elective METABOLIC EQUIVALENT 1-10 MET 1 MET Any procedure that which can safely be delayed for up to or over 1 year. Routine Preoperative Evaluation: There are many conditions which supercede routine perioperative risk evaluation for non-emergency surgery and require further stabilization prior to proceeding to the operating room this includes problems such as complete heart block, symptomatic bradycardia, an acute coronary syndrome etc... Several risk stratification models are in use but the most dominant is the revised cardiac risk index (RCRI) but several other models also are in use such as the ACS NISQP Surgical Risk Calculator (riskcalculator.facs.org) and the ACS NSQIP MICA Calculator: (surgicalriskcalculator.com/miorcardiacarrest). READING Revised Cardiac Risk Index 4 MET GARDENING 6 MET Composite Risk (%) 15 10 5 0 0 1 2 3+ Indicator of risk of suffering perioperative myocardial infarction, pulmonary embolism, VF or other cardiac arrest or complete heart block. JUMPING ROPE 8-10 MET 1) 2) 3) 4) 5) 6) High Risk Emergent surgery Vascular surgery Surgery with large EBL / fluid shift Intermediate Risk Carotid endarterectomy Head and neck surgery Intraperitoneal surgery Intrathoracic surgery Orthopedic surgery Prostate surgery CLIMBING STAIRS 7 MET Procedural Risk Class Low Risk Endoscopic procedures Superficial procedure Cataract surgery Breast surgery Coronary artery disease Cardiomyopathy History of TIA or stroke Insulin dependent diabetes Creatinine > 2.0 mg/dL Planned high risk surgical procedure American RUNNING Heart Association Lee et al. Circulation. 1999; 100(10): 1043-1049 PREOPERATIVE EVALUATION Algorithm for the risk evaluation of patients prior to surgery Patient scheduled for surgery with known or risk factors for CAD Nonemergent surgical case Clinically stable Emergent surgical case Clinical risk stratification and proceed directly to surgery Acute coronary syndrome Evaluate and treat accordingly Estimated perioperative risk of MACE based on combined clinical / surgical risk (RCRI etc...) Low risk (<1%) Elevated risk (>1%) Functional capacity <4 METs or unknown Functional capacity >4 METs Will further testing impact decision making or perioperative care? No further testing Yes No Proceed to surgery Pharmacologic stress testing with angiography and revascularization as indicated American Heart Association Proceed to surgery or alternate management as appropriate PREOPERATIVE CHECKLIST EKG Should be obtained in patients with known CAD/PAD, arrhythmia, cerebrovascular disease, or other significant structural disease except those undergoing low risk surgery. It should also be considered in patients without above risks factors except those undergoing low risk surgery Echocardiogram Pre-operative echo should be obtained in patients with dyspnea of unknown origin to assess LV function, known heart failure with change in clinical status, re-assessment of LV function in clinically stable patients with previously documented decreased LV function if there has been no assessment within a year. Stress Test Reasonable for patients at elevated risk for noncardiac surgery with poor functional capacity to undergo either exercise/dobutamine stress echo or myocardial perfusion scan if it will change management Please refer to stress testing and ACC pre-operative algorithm. Angiogram Routine preoperative coronary angiography is not recommended per ACC guidelines Beta Blockers Continue beta blockers in patients on them chronically. In patients with intermediate or high preoperative test (RCRI>3), it may be reasonable to begin beta blockers prior to surgery. Do not initiate beta blockers in the immediate pre-operative period (at least 2-7 days prior to surgery). Statins Continue statins in patients on them chronically. Consider initiating statins before vascular surgery or those with one clinical risk factor. Can consider initiating prior to elevated risk surgery in patients who already meet an indication for statin therapy. ACE / ARB Reasonable to continue perioperatively if already on them chronically. If held, restart as soon as safe following surgery. Antiplatelets Continue DAPT in patients undergoing urgent noncardiac surgery in the first 4 to 6 weeks after BMS or DES implantation, unless risks of bleeding outweigh risk of stent thrombosis. Patients with stents undergoing surgery that requires discontinuation of P2Y12 inhibitors, continue aspirin and restart P2Y12 inhibitor as soon as possible following surgery. In those undergoing nonurgent surgery and without prior stents, it may be reasonable to continue aspirin if patient at high risk of cardiac events and benefits outweighs risk of bleeding. CARDIAC IMAGING Modality Advantages Limitations / Contraindication Exercise ECG Ideal in low to intermediate risk patients who can exercise enough to get to target heart rate and have an interpretable ECG. Cannot use if patient has baseline LBBB or paced rhythm. Exercise should be attempted in any stress modality which its felt the patient can reasonably achieve target heart rate. Provides functional capacity. Cannot perform in a patient having ACS or recent MI, severe symptomatic AS, aortic dissections, or acute PE. Myocardial Perfusion Imaging Appropriate in wide range of pre-test probability patients. Balanced ischemia in triple vessel disease may lead to false negatives. Regadenoson or Adenosine Can assess viability Cannot use in pregnant patients, with hypotension (SBP<90), high degree AV block, active wheezing. Stress Echo No radiation exposure Dobutamine Appropriate in a wide range of pre-test probability patients. Can be limited by poor patient echo windows – obese patients, etc. Contraindicated in patients with recent ACS, tachyarrhythmias, uncontrolled hypertension (SBP>200/110), aortic dissections and large aneurysms. Can assess viability Can obtain additional hemodynamic data during stress. Stress MRI No radiation exposure GFR must be >30 Can assess for viability/scar ICD/Pacemakers limit study quality. Excellent structure/anatomy imaging Appropriate in intermediate and high pretest risk patents. Coronary CTA Can potentially end up with double contrast dye exposure if positive. Cannot use in symptomatic severe AS, aortic dissection, ACS. Option for low to intermediate risk patients with normal ECG and normal cardiac biomarkers. Requires high technical skill Needs optimal heart rate and should be able to participate in breath holds. No functional capacity is obtained. Contrast dye exposure Excellent tool for assessing anatomy of coronary arteries. Limited to larger caliber more proximal vessels. Patient will need to perform breath holds and have a controlled heart rate. NOT a stress test High negative predictive value Cannot use in pregnancy Cardiac Cath Optimal test for high pretest probability, positive stress tests, and those with acute coronary syndromes. Invasive with risks of complications such as bleeding Functional assessment with CT-FFR. Gold standard study for CAD. Contrast dye exposure SYNCOPE Reflex Syncope w Cerebral Hypoperfusion tu ic F a il u re Sec onda ry ANF no Poor Ve us Re Low Volume Va so d 80% 60% 40% 20% sP oo l om rn Lo ar m Pri yA NF t t on ia Card c Outp u scular Resista Va n ow mal Autonoics Nor L iac Syncope Card ce nh ib ctu ia Stru ral Othe thm r rhy Ar Pulmonar y) diac ( Car priate Refl ppro ex Ina Au n sio uced Dysautonom ten g Ind o ia p Dru sor res ep Car dI Etiology of Syncope (%) 100% Mixed u no Ve Orth o s t ati cH y Physiologic Basis of Syncope: Any mechanism which causes a transient drop in cerebral blood flow below 50-60 mL/min for at least 6-8 seconds can result in syncope. 0% <40 40-60 >60 Age (Years) Orthostatic Structural Disease Neurally Mediated Arrhythmia *Syncopized is not a word. Syncopated refers to accentuating the off beats in music. Neither refers to syncope. Don’t ever say it again. Orthostatic Hypotension: Drop in CBF because of either volume depletion, excess venous pooling or a failure of compensatory vasoconstriction. Further deliniation requires closer assessment of autonomic function and volume status. -Compression stockings -Fludricortisone -Medication review (may need to stop offending drugs like alpha blockers etc...) Reflex Syncope: Drop in CBF via sudden changes in efferent autonomic activity, especially parasympathetic, causing bradycardia and a release of sympathetic tone causing a drop in vascular resistance (in other words vasodilation). Look for common triggers like going to the bathroom or around the time of procedures (EGD, C-scope, cystoscopy, carotid massage etc...) or psychologic stress like seeing blood or extreme fear. -Increased salt and water intake -Counterpressure maneuvers -No data to support use of any medications except possibly beta blockers in patients over 40 Cardiac Syncope: Drop in CBF Pretty straight forward subgroup with either structural lesions (HOCM or severe AS) or arrhythmias as the mechanism. -Treatment of underlying process Initial Diagnostic Evaluation Orthostatic Vital Signs Echocardiogram with doppler EKG Telemetry Driving Restrictions Clearly documented that patient is restricted from driving if no immediately reversible cause is identified Renal 100% (1 min) Beta 1 receptor mediated increase in cardiac contractility and HR. Increases SVR via potent Alpha activity. Renal 100% (1 min) Beta 1 receptor mediated increase in cardiac contractility and HR. Increases SVR via potent Alpha activity. Epinepherine (β1 / β2 / α1) 1 - 20 mcg / min Norepinepherine (β1 / α1) 0.02 - 1.0 mcg / kg / min Beta 1 receptor mediated increase in cardiac contractility and HR. Decrease in SVR via Beta 2 mediated vasodilation. Renal ~80% (2.3 hrs) PDE3 - cGMP mediated increase in intracellular intracellular Ca++ concentration and consequent rise in contractility and CI. Decreases SVR / PVR. Primary Activity Renal (2 minutes) 0.20 - 0.50 mcg / kg / min Milrinone (cGMP / PDE3) Clearance (t½) Dobutamine (β1 ++ / β2 +) 2.5 - 10 mcg / kg / min Dose Range Agent (MOA) INOTROPIC AGENTS Dose Range 0.02 - 1.0 mcg / kg / min 1 - 20 mcg / min 0.03 units / min 1 - 10 mcg / kg / hr 0.5 - 20 mcg / kg / min Agent (MOA) Norepinephrine (β1 / α1) Epinephrine (β1 / β2 / α1) Vasopressin (V1) Phenylephrine (α1) Dopamine (β1 / α1) Primary Activity Renal 80% (2 min) Hepatic (2-3 hrs) Dose dependent effect. Alpha 1 and Beta 1 dominant activity at doses above 10 mcg / kg / min. Pure Alpha 1 mediated vasoconstriction Renal 10-15% (10-20 Vasopressin 1 receptor mediated min) vasoconstriction. Renal 100% (1 min) Beta 1 receptor mediated increase in cardiac contractility and HR. Increases SVR via potent Alpha activity. Renal 100% (1 min) Beta 1 receptor mediated increase in cardiac contractility and HR. Increases SVR via potent Alpha activity. Clearance (t½) VASOPRESSOR AGENTS 5 - 200 mcg / min 0.1 - 10 mcg / kg / min Onset: 15 - 30 min Duration: 6 - 12 hours Enalaprilat 1.25 mg – 5 mg Q6H 5 - 15 mg / hr +Δ 2.5 mg Q15 min Onset: 5 -15 min Duration: 1.5 - 4 hours Nicardipine Onset: 2 - 5 min Duration: 5 - 10 min Nitroglycerine Onset: 1 min Duration: 1 - 10 min Nitroprusside Onset: 5 -10 min Duration: 2 - 4 hours Labetalol 20 - 80 mg IVP Q10 min (max 300 mg) 10 - 20 mg IVP Hydralazine Onset: 10 - 20 min Duration: 1 - 4 hours Dose Range Agent First dose hypotension in high renin states, headache, dizziness Tachycardia, dizziness, flushing, nausea, headache, phlebitis, edema Reflex tachycardia, tachyphylaxis, nausea, headache, vomiting, flushing, methemoglobinemia Cyanide and thyocyanate toxicity, nausea, vomiting, muscle spasm, sweating, increased intracranial pressure Bronchospasm, heart block, nausea, paresthesias, dizziness Tachycardia, headache, flushing, nausea Adverse effects Pregnancy and acute renal failure Need an arterial line for monitoring. Decompensated heart failure. Need an arterial line for monitoring. Recent use of PDE-5 inhibitors (i.e Sildenafil). Need an arterial line for monitoring. Acute MI, significant CAD, stroke, increased ICP, renal or hepatic failure. Cocaine intoxication, decompensated heart failure, high grade AV blocks, significant bradycardia Increased intracranial pressure or glaucoma Contraindications IV ANTIHYPERTENSIVES Target Dose 50 mg PO TID 10 mg PO BID 40 mg PO QD 50-75 mg PO TID 20-80 mg PO TID 60-120 mg PO QD Agent (MOA) ACE Inhibitors Captopril Enalapril Lisinopril Vasodilators Hydralazine Isordil Imdur Angiotensin Receptor Blockers Valsartan Aldosterone Antagonist Spironolactone Beta Blockers Carvedilol Metoprolol XL (not Lopressor) Agent (MOA) HEART FAILURE AGENTS 80-160 mg QD - BID 25 mg PO QD 25 mg PO BID 200 mg PO QD Target Dose Nitroprusside 0.1 - 10 mcg / kg / min Hepatic / RBCs (1 - 3 Small vessel vasodilation primar- Stroke / TIA Coarctation min) ily afterload reduction. VSD Hepatic / RBCs (1 - 3 Converted to NO promoting Acute inferior MI HOCM min) venous (preload) relaxation. Small afterload reducing effect. Contraindications Nitroglycerine 5 - 200 mcg / min Primary Activity Clearance (t½) Agent (MOA) Dose Range VASODILATORS AGENTS Non-Valvular AF Non-Valvular AF: 5 mg PO BID but if two or more conditions apply (Cr >1.5, Age >80, wt< 60 kg) then 2.5 mg PO BID Apixaban (Eliquis) Factor Xa Inhibitor Onset: 1-2 hours Clearance: Renal 50% (12 hrs) Reversal: KCentra Edoxaban (Savaysa) Factor Xa Inhibitor Onset: 3-4 hours Clearance: Hepatic (12 hrs) Reversal: KCentra Non-Valvular AF GFR 95-50: 60 mg PO QD GFR 50-15: 30 mg PO QD DVT / PE Treatment 10 mg PO BID x 7 days then 5 mg PO BID GFR >15: 20 mg PO QD Factor Xa Inhibitor Onset: 2-3 hours DVT / PE Treatment Clearance: Renal 66% (5-9 hrs) GFR >15: 15 mg PO BID x 21d then 20 mg QD Reversal: KCentra Rivaroxaban (Xarelto) DVT / PE Treatment GFR >30: 150 mg PO BID Non-Valvular AF GFR >30: 150 mg PO BID GFR 15-30: 75 mg PO BID Dabigatran (Pradaxa) Direct Thrombin Inhibitor Onset: 90 min Clearance: Renal (12-27 hrs) Reversal: Praxbind Dosing Agent (MOA) Avoid using in patients with moderate to severe liver dysfunction. Avoid in patients with very high functioning kidneys due to excessivly rapid clearance. Avoid in severe hepatic impairment. Ok to crush and suspend in D5W 60 mL if given immediately. Avoid using in patients with moderate to severe liver dysfunction. Ok to crush tablets and mix with apple sauce. Avoid using with amiodarone, dronaderone, verapamil, ketoconazle, clarithromycin, quinidine. Must not open capsules. Considerations NOVEL ORAL ANTICOAGULANTS LANDMARK CLINICAL TRIALS Topic Trial Name (PMID) Brief Findings Atrial Fibrillation Anticoalguation Apixiban (Eliquis) Dabigatran (Pradaxa) Rivaroxiban (Xarelto) Rate Control Threshold Rate vs Rhythm Control ARISTOTLE (21870978) RE-LY (19717844) ROCKET-AF (21830957) RACE-II (20231232) AFFIRM (12466507) Apixiban superior to coumadin for stroke prevention (NNT 300) Dabigatran non-inferior to coumadin for stroke prevention Rivaroxiban non-inferior to coumnadin for stroke prevention Lenient HR control (<110) non-inferior to strict (<80 bpm) for risk of MACE Rate control non-inferior to rhythm control for risk of MACE Ischemic Heart Disease Antiplatelet and Anticoagulant Therapy ASA ISIS-2 (2899772) Clopidogrel CURE (11519503) Prasugrel TRITON TIMI 38 (17982182) Ticagrelor PLATO (20079528) Revascularization Paclitaxel DES in STEMI HORIZONS AMI (19420364) Compl. Revasc STEMI PRAMI (23991625) PCI in UA / NSTEMI RITA-3 (16154018) DES PCI vs CABG SYNTAX (19228612) FREEDOM (23121323) PCI vs OMT COURAGE (17387127) FAME-2 (22924638) Access Radial vs Femoral RIVAL (21470671) Heart failure Enalapril Valsartan Spironolactone Carvedilol Digoxin Ivabradine Dialysis with UF Hypertension Benazepril + Amlodipine Benazepril + HCTZ Lisinopril Amlodipine Chlorthalidone Losartan Valsartan ASA reduces reinfarct and death [very old study] (NNT 20) Addition of Clopidogrel to ASA reduces MACE (NNT 50) Prasugrel superior to clopidogrel with PCI (NNT 50) Ticagrelor superior to clopidogrel with PCI (NNT 60) DES in STEMI reduced TVR but not MACE (NNT 35) Non-infarct artery PCI reduces death / MI (NNT 7) PCI in high risk pts over OMT alone reduces 5 yr MACE (NNT 30) CABG in LM or 3v CAD superior to PCI (effect rises with SYNTAX score) CABG superior to PCI in diabetic pts to reduce MACE at 5 years (NNT 12) OMT non-inferior to BMS PCI for stable CAD for 5 year MACE FFR guided PCI v OMT in stable CAD reduced urg revasc but not MI or death Radial approach reduced hemorrhagic complications (NNT 500) CONSENSUS (2883575) SOLVD (1463530) ValHeFT (11759645) RALES (10471456) COMET (12853193) Dig (9036306) BEAUTIFUL (18757088) SHIFT (20801500) CARRESS-HF (23131078) Enalapril in NYHA class IV reduced death(NNT 6) Enalapril in NYHA class II+ reduced hospitalization, not death (NNT 25) Valsartan in NYHA class II+ reduced hospitalizatoin (NNT 25) Spironolactone in NYHA class III+ reduced death(NNT 9) Carvedilol superior to Mortality in NYHA class II+ in reducing death (NNT 18) Digoxin in Systolic HF reduced hospitalization but not death. Ivadribine in stable CAD + HR >70 reduced ACS admits (NNT 50) Ivadribine in stable CAD + HR >70 reduced ACS and Death (NNT 20 / 50) Ultrafiltration “inferior” really equivalent to diuresis in NYHA class IV ACCOMPLISH (19052124) ACCOMPLISH (19052124) ALLHAT (12479763) ALLHAT (12479763) VALUE (15207952) ALLHAT (12479763) LIFE (11937178) VALUE (15207952) Benazepril+CCB reduced death / MI v Benazepril+HCTZ (NNT 50) See above Amlodipine v Chlorthalidone v Lisinopril all equal for ACS risk See above Amlodipine reduced MI but not mortality compared to Valsartan (NNT 140) See above Losartan reduced risk of stroke but not death or MI vs atenolol (NNNT 50) See above Trans-Catheter Aortic Valve Implantation (TAVI) TAVI in Surg High Risk PARTNER (22443479) Stroke and MI similar in both arms at 2 years. Dyslipidemia Primary prevention Rosuvastatin Secondary Prevention Rosuvastatin Atorvastatin Ezetimibe Niacin JUPITER (18997196) Rosuvastatin in patients with CRP >2 mg/L reduced MACE (NNT 150) SATURN (22085316) PROVE-IT (15007110) IMPROVE-IT (18376000) AIM-HIGH (22085343) Rosuvastatin 40 and Atorvastatin 80 both promoted atheroma regression Atorvastatin reduced death / repeat ACS compared to Pravastatin (NNT 25) Ezetimibe +Simvastatin in FHL did not reduce CIMT over Simvastatin alone. No benefit to addition of Niacin to Statin, trend toward increase stroke. *The above information is only an extremely condensed version of the full trial details. Please see the full paper for further details. Y CA G AM E Heart GE LE Association OF CAN COL RI American R DIOLO